Separation of organic compounds from mixtures by vapor permeation or pervaporation
We reviewed various membrane materials for vapor permeation of volatile organic compounds highlighting the scarcity of information on binary VOC/N2 mixtures and rare examination of multicomponent mixtures. Our experimental and theoretical research focus on the applicability of solubility parameters in predicting VOC/N2 separation efficiency, scoring best with Hansen solubility parameters for VOCs with limitations for cyclic hydrocarbons. We resolved the aging of thin film composite membranes exposed to alkane vapors facilitating conventional solvent exchange drying for membrane regeneration. Furthermore, we identified the primary contributors to errors in vapor permeability measurement, emphasizing the importance of optimizing operational parameters to reduce theoretical errors, beneficial for low-permeable membranes.
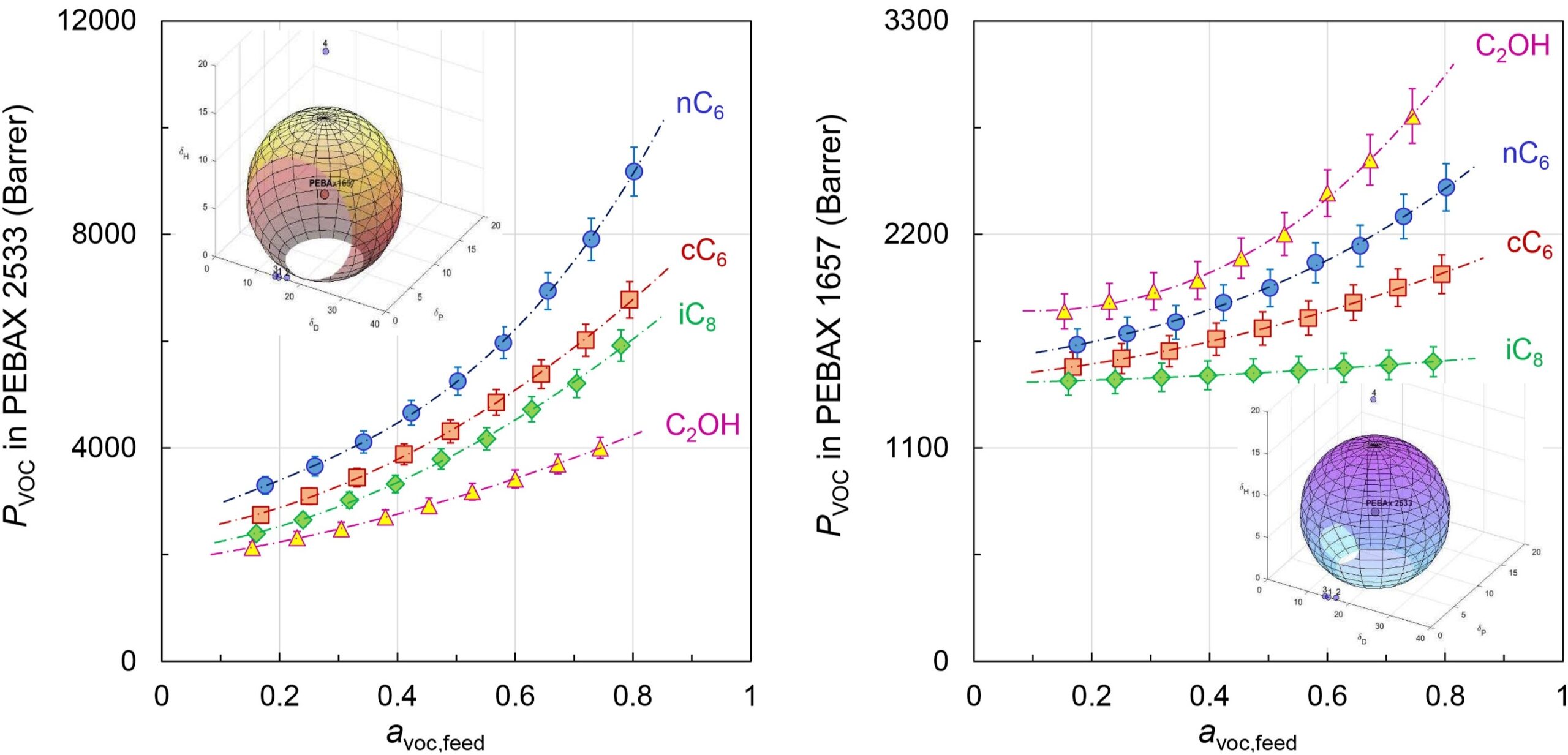
Vapor permeability of structurally different VOCs vs their activity in the feed including the particular Hansen solubility parameter 3D plot
- Jirsakova K., Stanovsky P., Dytrych P., Moravkova L., Pribylova K., Petrusova Z., Jansen J. C., Izak P.: Organic vapour permeation in amorphous and semi-crystalline rubbery membranes: Experimental data versus prediction by solubility parameters. J. Membr. Sci. 627(1 Jun), 119211, 2021. DOI
- Petrusová Z., Morávková L., Stanovský P., Machanová K., Koštejn M., Jandová V., Izák P.: Regeneration of thin-film composite membrane used for permeation of hexane vapors. Sep. Purif. Technol. 224(1 Oct), 62-69, 2019. DOI
- Petrusova Z., Machanova K., Stanovsky P., Izak P.: Separation of organic compounds from gaseous mixtures by vapor permeation. Sep. Purif. Technol. 217(15 Jun), 95-107, 2019. DOI
- Dytrych P., Vajglová Z., Morávková L., Jandová V., Izák P., Petrusová Z.: Minimization of the Theoretical Error of Input Parameters for a Vapor Permeation Apparatus Chem. Eng. Technol. 41(9), 1727-1736, 2018. DOI
Our research explored the application of pervaporation for separating organic compounds from mixtures. We successfully utilize pervaporation in conjunction with a Ru-BINAP chiral catalytic complex, avoiding catalyst sorption into the membrane and employing an ionic liquid/methanol phase as a green solvent for the reaction. Further, we successfully utilize the pervaporation of an azeotropic mixture with trimethyl borate, a precursor to boronic acids used in Suzuki couplings, using commercial membranes. Finally, our testing showed the potential for dealcoholizing beer using pervaporation with commercially available Sulzer membranes.
- Gaalova J., Hejda S., Stavarek P., Sykora J., Fajgar R., Kluson P., Izak P.: Pervaporation of (R)/(S)-methyl 3-hydroxybutyrate (Sigma MHB) from a mixture containing an ionic liquid, methanol and Ru catalyst. Sep. Purif. Technol. 222(1 Sep), 45-52, 2019. DOI
- Gaalova J., Vojtek L., Lasnier S., Tadic T., Sykora J., Izak P.: Separation of Trimethyl Borate from a Liquid Mixture by Pervaporation. Chem. Eng. Technol. 42(4), 769-773, 2019. DOI
- Halama R., Broz P., Izak P., Kacirkova M., Dienstbier M., Olsovska J.: Beer dealcoholization using pervaporation. Kvasny Prumysl 65(2), 65-71, 2019. DOI