Chiral membranes prepared by modification of anion exchange membranes sulfobutyl ether-β-cyclodextrins by ion interactions
Enantiomers are substances composed of the same number and type of atoms that are mirror images of each other. Enantiomers have practically identical physicochemical properties, but their reactions with other chiral substances can differ fundamentally. Several enzymes and receptors involved in biochemical processes show stereoselectivity. For this reason, the recognition and separation of enantiomers is significant, especially in the pharmaceutical industry, where individual drug enantiomers can exhibit completely different pharmacological effects. Membrane processes could be a potentially efficient, easily scalable enantioseparation technology to help produce safer and more effective enantiomerically pure drugs.
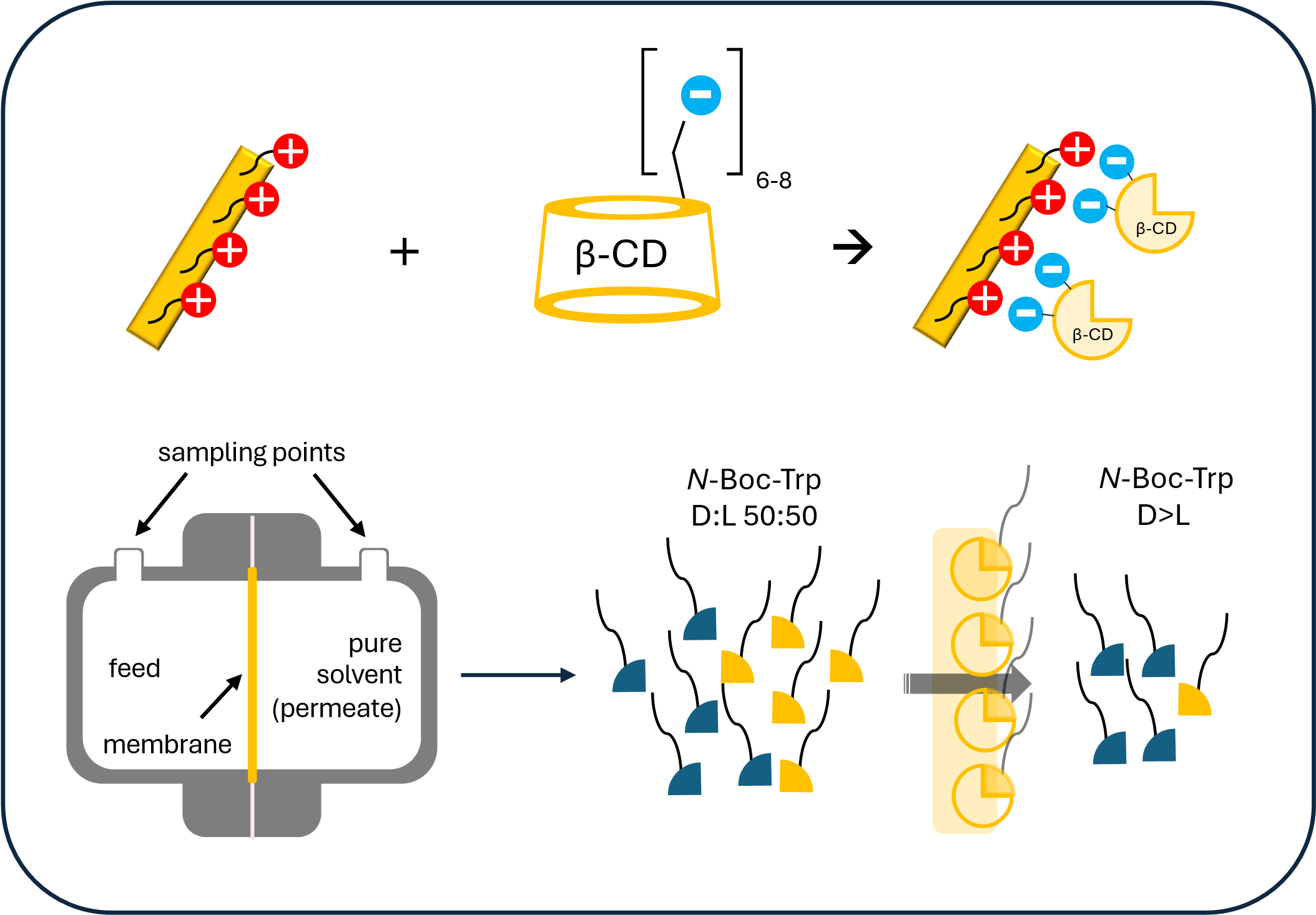
The preparation of enantioselective membranes involves incorporating chiral selectors capable of recognizing and selectively capturing one of the enantiomers of the studied substance. However, it is usually very complicated and costly, hindering their application on an industrial scale. This work demonstrates a simple method for preparing chiral membranes using ionic interactions between a negatively charged chiral selector and a positively charged membrane. Anion-exchange membranes were functionalized by a commercially available polyanionic chiral selector, sulfobutyl ether-β-cyclodextrin (SBE-β-CD). The combination of a powerfully charged selector and a membrane with quaternary ammonium groups enabled a stable and, at the same time, reversible modification of the surface and partially the internal structure of the non-porous membrane. The separation properties of the resulting chiral membrane were tested in diffusion cells for the separation of N-Boc-tryptophan as a model analyte. We studied various experimental conditions, including feed concentration, temperature, and solvent composition. The separation mechanism can be described as sorption-selective, with N-Boc-L-Trp preferentially adsorbed by forming inclusion complexes with cyclodextrins. Sorption generally decreases as the methanol content of the solvent increases. Both low temperature and low feed concentration also favor the selectivity of diffusion. The membrane can be regenerated by desorption of the analyte in pure methanol while maintaining its function. It can, therefore, be used repeatedly in the cyclic separation process.
The study of the Research Group of Membrane Separations was done in cooperation with the Institute of Organic Chemistry of the University of Chemistry and Technology in Prague, the Institute of Physical Chemistry of the University of Chemistry and Technology, and the Technical University of Liberec within the framework of the project 23-06152S supported by the Grant Agency of the Czech Republic. The Internal Grant Agency of the ICPF also funded the study in support of doctoral students’ research in 2023.
- Čížek J., Jandová V., Stanovský P., Hovorka Š., Yalcinkaya F., Kohout M., Izák P.*: Chiral membranes prepared by ionic interactions between sulfobutylether-β cyclodextrin and anion-exchange membranes. J. Membr. Sci. 2025, 717(February), 123592. doi.org/10.1016/j.memsci.2024.123592