The research team of Dr. Karban has long been focusing on the synthesis of fluorinated carbohydrates and their use as probes in biochemical processes involving carbohydrates. This research also covers recognition of fluorinated carbohydrates by carbohydrate-binding proteins, their use as inhibitors of carbohydrate-involving transformations, and the study of their effects on living cells including cytotoxicity. Cellular cytotoxicity is the subject of the presented publication, which reports the research conducted in cooperation with Masaryk Memorial Cancer Institute in Brno and the University of Chemistry and Technology in Prague. The results were recently published in the journal Organic & Biomolecular Chemistry.
The study builds on earlier reports that the introduction of fluorine into amino sugars can result in cytotoxic monosaccharides and that acylated non-fluorinated amino sugar hemiacetals (= derivatives with the unprotected anomeric hydroxyl) are also cytotoxic. A combination of these findings prompted us to prepare a set of acylated fluorinated N-acetyl-galactosamine (GalNAc) and -glucosamine (GlcNAc) hemiacetals. These compounds include all three structural features contributing to cytotoxicity: fluorination, acylation at the non-anomeric positions, and an unprotected anomeric hydroxyl. It was found that fluorination increased the cytotoxicity of amino sugar hemiacetals in most cases. This effect was most pronounced for GalNAc fluoro analogues. While the non-fluorinated acetylated GalNAc hemiacetal had very low cytotoxicity (IC50 = 414 µM), multiple fluorination increased the cytotoxicity up to IC50 = 20 µM against the aggressive triple negative breast tumor cells MDA-MB-231.
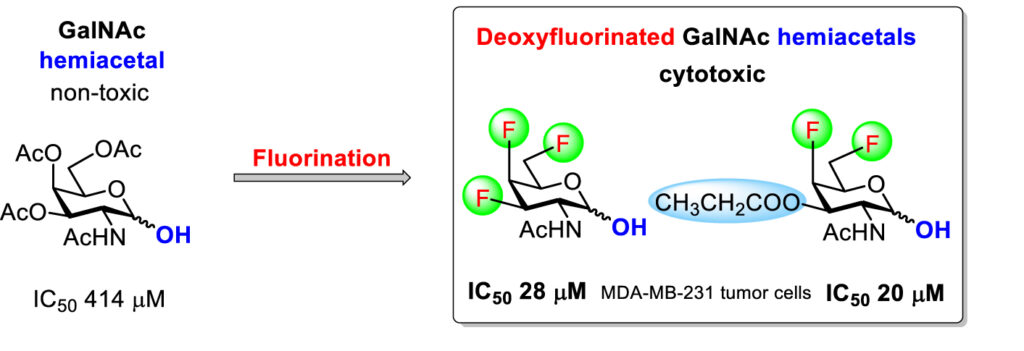
- Hamala, V.; Červenková Šťastná, L.; Kurfiřt, M.; Cuřínová, P.; Balouch, M.; Hrstka, R.; Voňka, P.; Karban, J., The effect of deoxyfluorination and O-acylation on the cytotoxicity of N-acetyl-d-gluco- and d-galactosamine hemiacetals. Org. Biomol. Chem. 2021, 19, 4497–4506. http://dx.doi.org/10.1039/D1OB00497B
In a parallel study, we developed the synthetic methods for the introduction of multiple fluorine substituents into amino sugars GlcNAc and GalNAc. It was imperative to overcome complications resulting from the instability of amino sugars during synthetic operations. In the end, the use of phenyl 2-azido-2-deoxy-thioglycosides as key intermediates was found advantageous. This enabled us to prepare a complete series of GlcNAc and GalNAc analogues deoxyfluorinated at all possible non-anomeric positions.
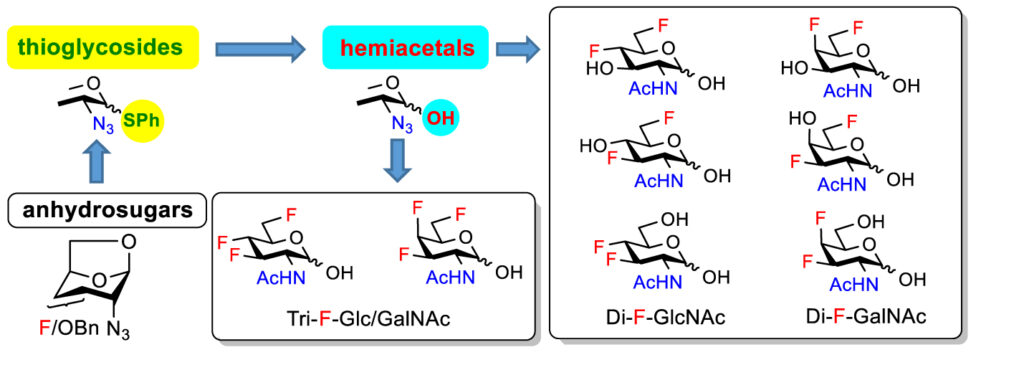
- Hamala, V.; Červenková Šťastná, L.; Kurfiřt, M.; Cuřínová, P.; Dračínský, M.; Karban, J., Synthesis of multiply fluorinated N-acetyl-d-glucosamine and d-galactosamine analogs via the corresponding deoxyfluorinated glucosazide and galactosazide phenyl thioglycosides. Beilstein J. Org. Chem. 2021, 17, 1086–1095. https://doi.org/10.3762/bjoc.17.85