Experimental and numerical study of the flux of isobutane vapors near saturation through multi-layered ceramic membranes
Capillary condensation of vapors in small pores can significantly affect the efficiency of gas separation through a porous membrane. It occurs under pressure conditions close to the saturated vapor pressure of the condensable gas. During the condensation of one component of a separated mixture, the transport of not only this component (condensable gas) changes but also the flow of the permanent (non-condensable) gas through membrane pores is significantly affected. However, this separation mechanism is still not fully understood and only rarely imperfect descriptions can be found in the literature. This process is interesting not only from a theoretical point of view, but it can also has practical applications. The direction of transport of the condensable gas through the multilayer material (direction from larger pores to smaller ones or vice versa) can significantly affect the mass flow through the porous membrane. This effect was first described in the work of Dr. Uchytil in 1994 (https://doi.org/10.1016/0376-7388(94)00157-T).
Dr. Uchytil and his colleagues have been dealing with the condensation of gases during transport through a multilayered porous medium for a long time. Since 2006, they have been collaborating on this topic with Dr. Loimer from the Technical University in Vienna, with whom they published several publications in prestigious international journals. The transport of vapors of isobutane near saturation during permeation through multi-layered asymmetric membranes (3 to 5 layers with a pore size of 3 mm to 20 nm) was investigated experimentally and theoretically in the latest work published in the renowned scientific journal Separation and Purification Technology. The influence of the upstream state of the vapor, whether far or close to saturation, and of the orientation of the membrane on the mass flow rate was investigated. For a membrane with five layers, the mass flux increased by about 80% for a vapor close to saturation. Also, close to saturation the mass flux in the flow direction from the separation layer to the support was up to 50% larger than in the opposite direction. Three theoretical descriptions of the flow process were given in the work, assuming that condensation may take place, with respect to heat transfer from the surroundings. For one description any heat transfer was neglected and the flow was assumed to be isothermal while for two other descriptions heat transfer and temperature variations due to condensation and evaporation were considered. Qualitatively, the increase of the mass flux for a vapor close to saturation and the dependence of the mass flux on the flow direction was recovered by all three descriptions.
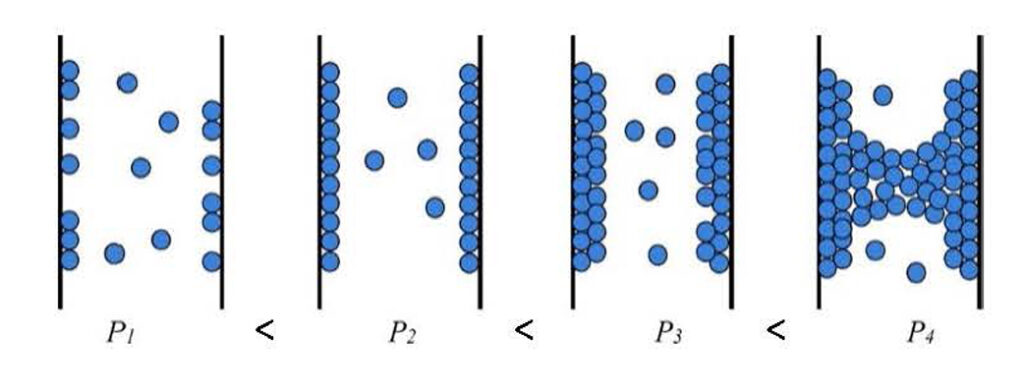
Schematic diagram of the dynamics of capillary condensation in pores for a single-component vapor with increasing pressure. First, adsorption takes place on the pore walls, as the relative pressure increases, adsorption occurs in multiple layers until the layers are connected and a meniscus of condensed steam is formed
- Setničková K.,* Petričkovič R., Uchytil P., Loimer T.: Experimental and numerical study of the flux of isobutane vapors near saturation through multi-layered ceramic membranes. Separation and Purification Technology 2023, 306, 122604. doi: 10.1016/j.seppur.2022.122604