The GACR project entitled “Development of new organic-inorganic composite materials based on dendrimers as effective gas and vapour sorbents” was rated ‘Excellent’
Bilateral project to promote international cooperation in basic research based on signed international cooperation agreements between the GACR (Czech Science Foundation) and the National Science and Technology Council (NSTC), Taiwan. The collaborating partners in this project were the Institute of Chemical Processes of the CAS (project investigator Kateřina Setničková) and Chung Shan Medical University in Taichung (project investigator Prof. Hui-Hsin Tseng).
Anthropogenic carbon dioxide is considered one of the main causes of the greenhouse effect responsible for global warming. To reduce greenhouse gas emissions, it is necessary to develop new technologies for energy production with minimal CO2 emissions and, at the same time, to search for new methods of carbon capture and storage (CCS). Kateřina Setničková has been working for a long time on the removal of CO2 from gas mixtures, mainly using membrane technologies, and in recent years has also focused on the adsorption of gases in materials. This process is considered to be an efficient alternative to CO2 capture, offering potential energy savings compared to other established separation processes.
The project aimed to carry out research focused on the development of new composite materials and systems for efficient capture/evaporation of gases and vapors, especially CO2. The key tasks were 1) synthesis of dendritic materials, preparation of carriers and composite materials with suitable sorption properties and their characterization, and 2) design and construction of experimental equipment for sorption and permeation measurements needed to evaluate the separation efficiency of the prepared materials.
Although the collaboration between the two laboratories was effectively limited to more than 2 years due to the Covid-19 pandemic and both teams were forced to work more or less independently, interesting results were obtained and published in prestigious scientific journals.
Within the project, a range of materials for CO2 separation were prepared and tested:
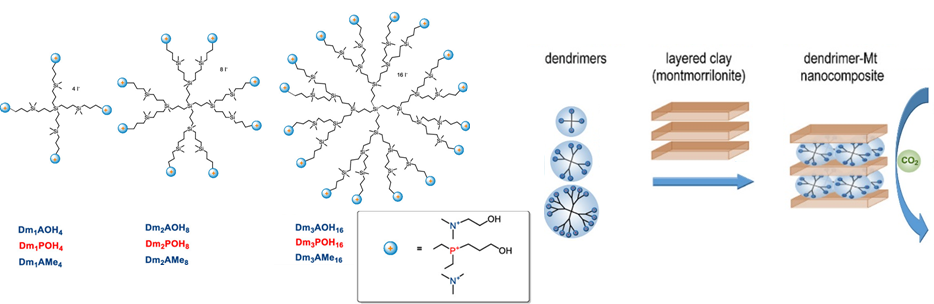
- Montmorillonite-based layered silicates functionalized by dendritic modifiers (ACS Sustainable Chemistry & Engineering 2020, 8, 11692-11703. DOI: 10.1021/acssuschemeng.0c03367, Q1).
For all materials, the CO2 adsorption capacity as well as the CO2/CH4 and CO2/N2 selectivity increased (up to 200%) after modification with dendrimers. However, the adsorption capacity of the montmorillonite-based material was lower (< 1 mmol/g) compared to other materials studied as CO2 adsorbents.
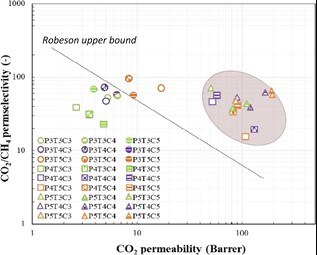
- CMS (carbon molecular sieve) – materials based on carbon molecular sieves with excellent parameters for CO2 separation. The use of CMS in the form of a thin selective layer of multilayer inorganic membranes proved to be a very promising application for effective CO2/CH4 separation. The performance of the prepared CMS materials exceeded the Robeson limit representing an indicator of membrane quality (Separation and Purification Technology 2021, 269, 118627-118636, DOI: 10.1016/j.seppur.2021.118627, D1).
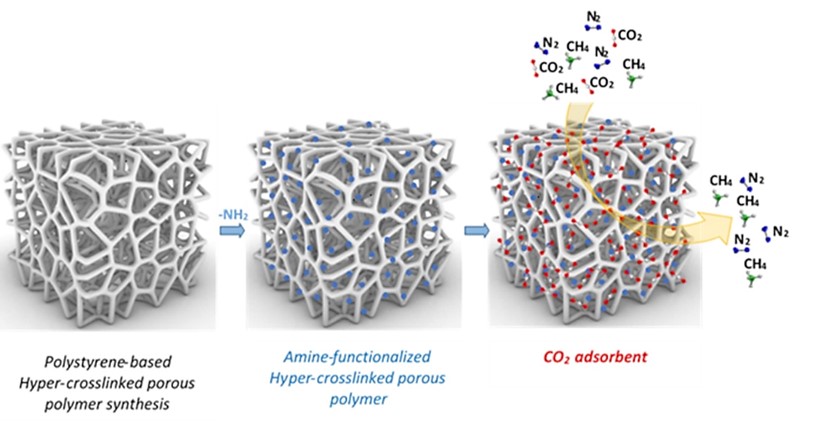
- Cross-linked porous polymers based on styrene, modified by amino groups (amines, dendrimer), showed a large internal surface area, which decreased slightly after functionalization, but their selective adsorption to CO2 compared to CH4, N2 and H2 increased significantly (Polymers 2023, 15, 13. DOI: 10.3390/polym15010013, Q1).
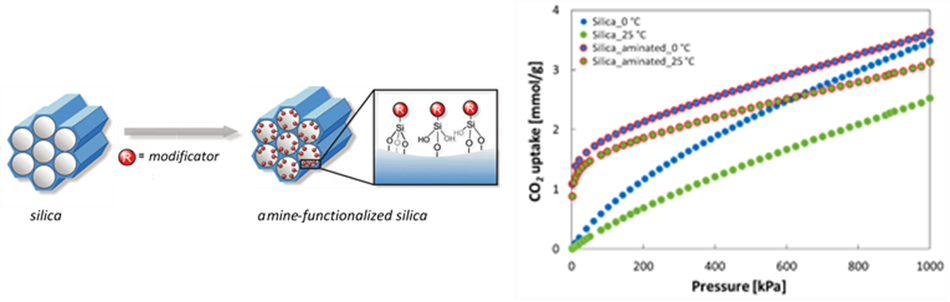
- Silica materials modified by amino silanes (structure similar to dendrimer) and dendrimers exhibited a large internal surface area that was reduced after amination, but the CO2 adsorption capacity increased up to 4-fold after amination at atm. pressure and 25 °C. So this material appears to be very promising for CO2 capture (publication in preparation).
- To determine the sorption of gases and vapors in different matrix materials and to promptly evaluate the separation performance of the systems, we have designed several unique devices and original procedures (Journal of Membrane Science 2022, 661, 120867. DOI: 10.1016/j.memsci.2022.120867, D1; Separation and Purification Technology 2023, 306, 122604. DOI: 10.1016/j.seppur.2022.122604 , D1; Chemical Engineering Journal 2022, 450, 138233. DOI: 10.1016/j.cej.2022.138233, D1).
Illustration of the principle of evaluating gas sorption in a polymer membrane based on a permeation experiment
The main difficulties in the project were related to the modification of materials with dendrimers. Dendrimers are spatially demanding molecules, which is the reason for their limited interaction with the matrix. A further complication is the possibility of dendrimer binding only via terminal functional groups targeted specifically for CO2 adsorption. A possible way to address these issues in future research on this topic seems to be the application of dendrons (dendritic wedges), which are much smaller and can bind to the support via the core atom, thus not reducing the utility of the functional groups as binding sites for the reaction with CO2.