Stereoselectivity in glycosylation with fluorinated carbohydrates
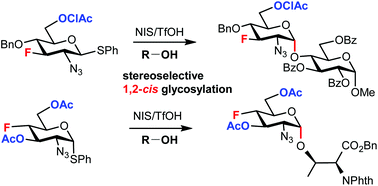
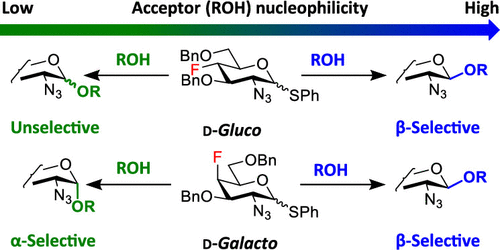
The stereoselective formation of a glycosidic bond remains the main challenge associated with the chemical synthesis of complex glycostructures. The influence of substituents at remote positions of the pyranose ring on the stereoselectivity of glycosylation is increasingly recognized. We study how fluorine substituents at C3, C4, and C6 of 2-azido-hexopyranosyl donors affect the stereoselectivity in glycosylation. We use fluorinated thioglycoside donors prepared from our recently synthesized fluorinated 2-azido-1,6-anhydro-hexopyranoses. Their stereoselectivities are compared to those of the corresponding O-benzylated and O-acetylated analogs and the mechanistic interpretation is suggested. Using a modification of both glycosyl donors and acceptors and varying reaction conditions, we are able to develop procedures for 1,2-cis glycosylation with deoxofluorinated 2-azido-2-deoxy-d-gluco/galactopyranosyl donors.
 |
 |
 |
- M. Kurfiřt, L. Červenková Št’astná, P. Cuřínová, V. Hamala, J. Karban: J. Org. Chem. 86(7), 5073–5090, 2021. DOI
- V. Hamala, L. Červenková Št’astná, M. Kurfiřt, P. Cuřínová, M. Dračínský, J. Karban: Org. Biomol. Chem. 18(28), 5427–5434, 2020. DOI
- M. Kurfiřt, L. Červenková Št’astná, M. Dračínský, M. Müllerová, V. Hamala, P. Cuřínová, J. Karban: J. Org. Chem. 84(10), 6405–6431, 2019. DOI