Synthesis and cytotoxicity of multiply fluorinated carbohydrates
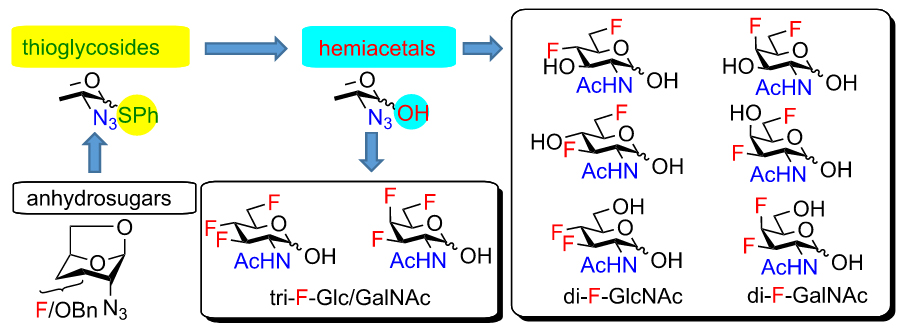
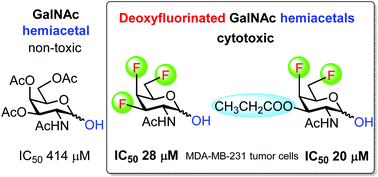
We have prepared a complete series of mono-, di- and trifluorinated analogs of N-acetylglucosamine and N-acetylgalactosamine. Most of them were obtained using 1,6-anhydropyranose chemistry for stereoselective introduction of fluorine at the 3- and 4-positions. The application of fluorinated 2-azido-1-thioglycoside intermediates permitted chemoselective manipulation of the anomeric position. We used this approach for the preparation of fluorinated acylated 2-acetamido-lactols, which exhibited cytotoxicity in the concentration of low tens of micromole.
 |
 |