Preparation of new phenacene derivatives
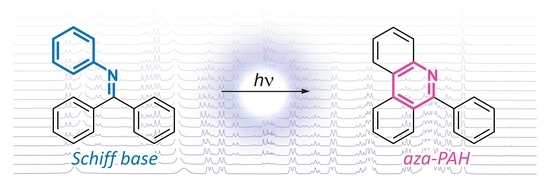
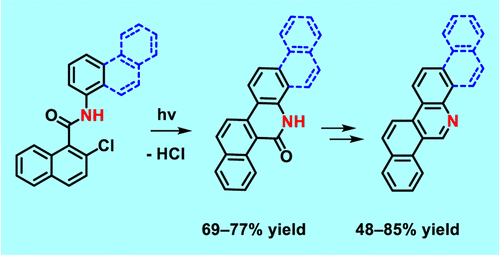
Phenacenes are known as stable compounds with resistance to oxygen and light, which makes these planar π-conjugated compounds suitable for utilization as functional materials in numerous applications. The introduction of nitrogen atoms into the phenacene skeleton leads to the formation of a dipole moment which is reflected in modified physicochemical properties when compared to their carbo analogs. Recently, a novel methodology for the synthesis of aza[n]phenacenes (n = 4 – 6) was successfully developed utilizing photocyclodehydrochlorination reaction of starting 2-chloro-N-aryl-1-naphthamides. Also, a novel N,N-didodecyl-3,10-diazapicenium salts with bromide and hexafluorophosphate counterions were synthesized as an exfoliant, a stabilizer, and a p-dopant for liquid phase exfoliated graphene.
 |
 |
 |