Determination of mercury behaviour during combustion processes
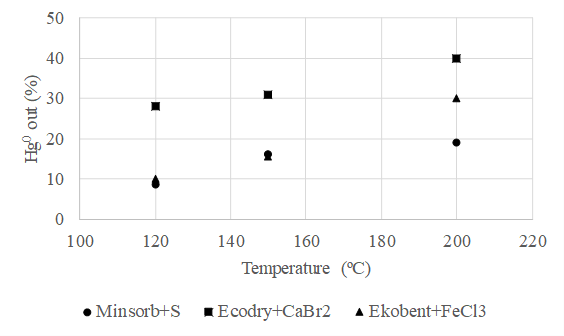
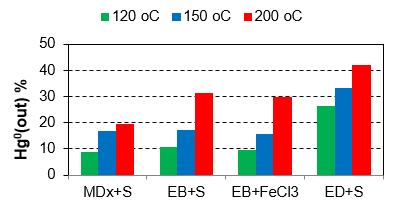 Mercury is one of the most significant pollutants in terms of emissions from coal combustion. For its effective capture, it is necessary to have a knowledge of its speciation. In the frame of extensive experimental activity, the behaviour of mercury during combustion was clarified and the key factors influencing its speciation were determined, and possible mercury capture methods were experimentally verified. There are three important factors for the effective sorption of mercury (esp. in the form of the vapour of elementary mercury Hg0):
Mercury is one of the most significant pollutants in terms of emissions from coal combustion. For its effective capture, it is necessary to have a knowledge of its speciation. In the frame of extensive experimental activity, the behaviour of mercury during combustion was clarified and the key factors influencing its speciation were determined, and possible mercury capture methods were experimentally verified. There are three important factors for the effective sorption of mercury (esp. in the form of the vapour of elementary mercury Hg0):
- specific sorbent surface (should be, if possible, more than 200 m2/g),
- sorption temperature (suitable to avoid temperatures above 150 °C),
- and impregnation (oxidizing compounds with chlorides and bromides, and agents with sulfur, in elementary form as well as polysulfides).
 During the sorption of Hg0 and mercury compounds on ashes, the decisive factors are the content of unburned carbon, chloride content, specific surface area and sorption temperature. The content of water vapour usually slightly hinders the sorption of mercury on sorbents. At higher ratios of HCl/SO2 concentrations in the flue gas and oxygen content above approx. 4 vol. %, Hg0 is oxidized in the gas to HgCl2. The oxidation depends on the temperature, catalytically active surface, and reaction time. Organometallic mercury compounds are transformed in the combustion processes to a mixture of Hg0 vapour and inorganic compounds (esp. HgCl2). In flue gas with a very low content of VOC, almost no organometallic compounds are formed. When mercury is to be removed by wet methods together with SO2, it is necessary to pay attention to the so-called re-emission of mercury caused by the reduction of Hg2+ to Hg0 with subsequent volatilization into the flue gas. These findings were published in a number of original works and serve as a base for the development of capture technology for mercury in flue gas.
During the sorption of Hg0 and mercury compounds on ashes, the decisive factors are the content of unburned carbon, chloride content, specific surface area and sorption temperature. The content of water vapour usually slightly hinders the sorption of mercury on sorbents. At higher ratios of HCl/SO2 concentrations in the flue gas and oxygen content above approx. 4 vol. %, Hg0 is oxidized in the gas to HgCl2. The oxidation depends on the temperature, catalytically active surface, and reaction time. Organometallic mercury compounds are transformed in the combustion processes to a mixture of Hg0 vapour and inorganic compounds (esp. HgCl2). In flue gas with a very low content of VOC, almost no organometallic compounds are formed. When mercury is to be removed by wet methods together with SO2, it is necessary to pay attention to the so-called re-emission of mercury caused by the reduction of Hg2+ to Hg0 with subsequent volatilization into the flue gas. These findings were published in a number of original works and serve as a base for the development of capture technology for mercury in flue gas.