The use of fluorinated oligosaccharides as probes for galectins
G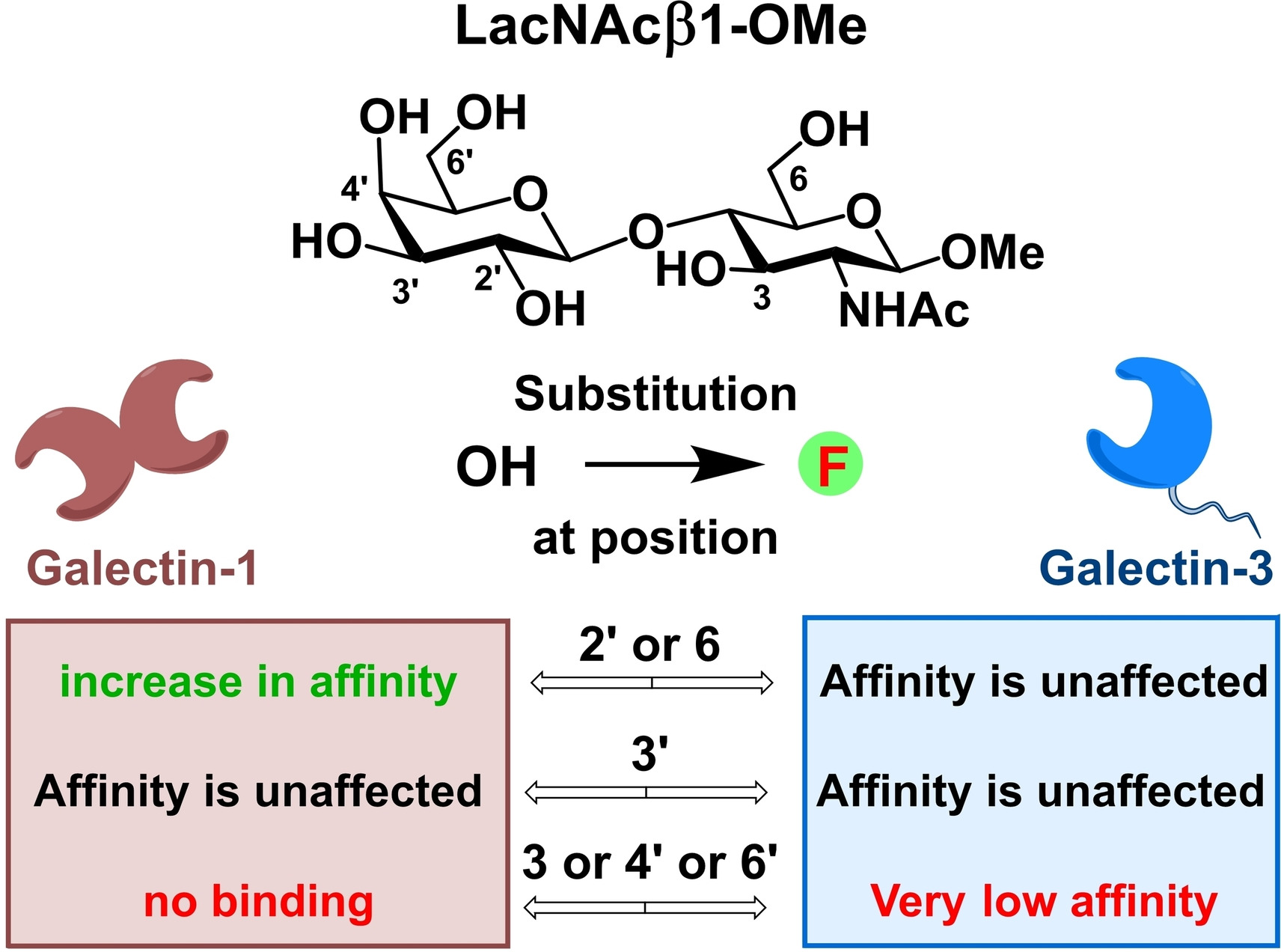 alectins are soluble proteins, which non-covalently bind β-galactosides. Non-covalent interactions of galectins with oligosaccharides containing this structural motif are implied in pathologies including cancer, inflammation, and fibrotic diseases. We synthesize fluorinated analogs of galectin ligands including disaccharides lactose, N-acetyllactosamine, and N,N’-diacetyllactosamine, and use them as probes for a panel of human galectins. In addition to the chemical mapping of the binding site, we also employ 19F NMR methods to elucidate the molecular basis of the observed binding phenomena. The attachment of monovalent fluorinated disaccharides to multivalent carriers (e.g. dendrimers and proteins) enables us to assess the impact of clustered presentation on affinity and selectivity. Modulation of the ligand selectivity to galectins by deoxyfluorination and ligand clustering is expected to lead to the identification of selective inhibitors. Fluorinated tetrasaccharides formed by β-(1-4) glycosidic attachment of selected fluorinated disaccharides to natural or fluorinated lactose or N-acetyllactosamine is currently employed to map the less conserved binding subsites A and B, whose binding preferences towards glycomimetics are mostly unknown.
alectins are soluble proteins, which non-covalently bind β-galactosides. Non-covalent interactions of galectins with oligosaccharides containing this structural motif are implied in pathologies including cancer, inflammation, and fibrotic diseases. We synthesize fluorinated analogs of galectin ligands including disaccharides lactose, N-acetyllactosamine, and N,N’-diacetyllactosamine, and use them as probes for a panel of human galectins. In addition to the chemical mapping of the binding site, we also employ 19F NMR methods to elucidate the molecular basis of the observed binding phenomena. The attachment of monovalent fluorinated disaccharides to multivalent carriers (e.g. dendrimers and proteins) enables us to assess the impact of clustered presentation on affinity and selectivity. Modulation of the ligand selectivity to galectins by deoxyfluorination and ligand clustering is expected to lead to the identification of selective inhibitors. Fluorinated tetrasaccharides formed by β-(1-4) glycosidic attachment of selected fluorinated disaccharides to natural or fluorinated lactose or N-acetyllactosamine is currently employed to map the less conserved binding subsites A and B, whose binding preferences towards glycomimetics are mostly unknown.
- M. Kurfiřt, M. Dračínský, L. Červenková Šťastná, P. Cuřínová, V. Hamala, M. Hovorková, P. Bojarová, J. Karban: Chem. Eur. J. 27(51), 13040–13051, 2021. DOI