Design, synthesis and study of polycyclic aromatic compounds
[n]Helicenes and [n]phenacenes, polycyclic aromatic compounds which are derived from the structure of 2D graphene, have semiconductive characteristics. Both types of molecules are comprised of ortho-condensed benzene rings; while [n]helicenes form a screw-shaped non-planar helix, aromatic rings of [n]phenacenes have a “zig-zag” topology, and the resulting structures are linear. Our group is interested in the design, synthesis, and study of the application potential in optoelectronics and sensing.
Preparation of functional thin layers
Along with studying the properties of polycyclic aromatics, thin layers are prepared and adapted to electronic components. Thin layers of well-soluble derivatives can be fabricated using simple solution methods; less soluble compounds are subjected to different deposition techniques from a gas phase. We have also developed a unique methodology of deposition of these aromatics through electropolymerization.
Other research topics
- Separation of helicene enantiomers
- Synthesis and research of steroids
- Synthesis and research of cannabinoids
- Chemical transformation of g-C3N4
- Photooxidative cyclization using ultra-fast spectroscopy
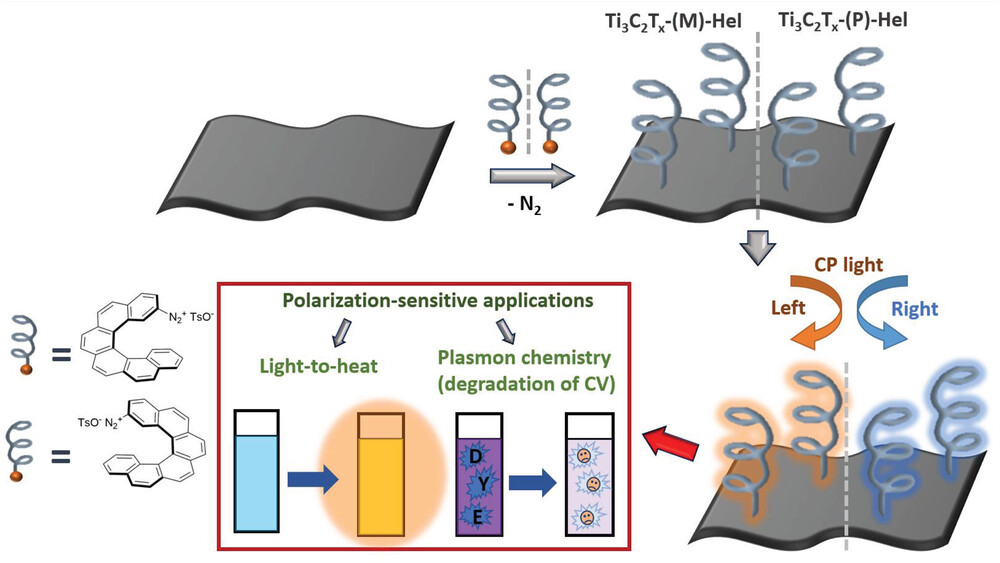
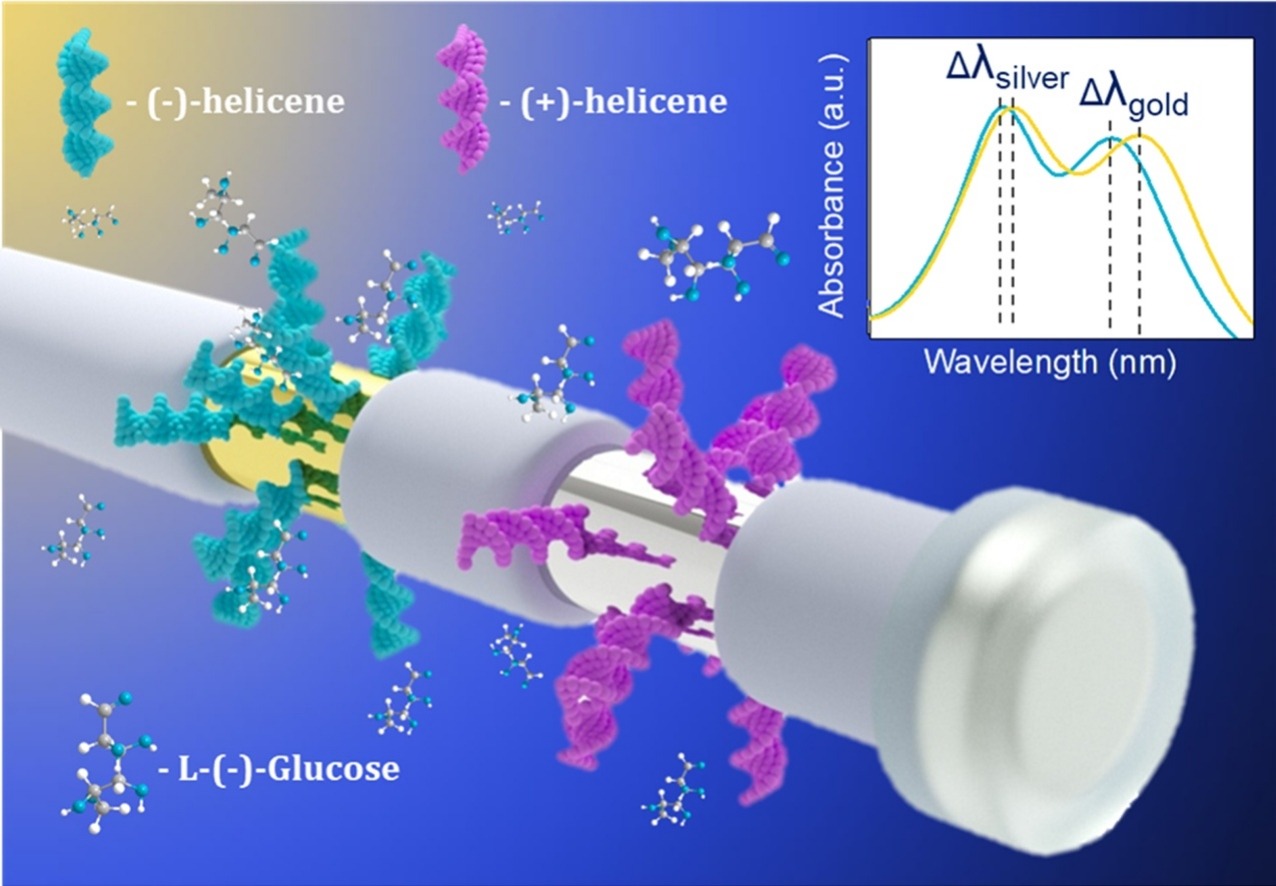
Chiral Plasmonics
A special place in our research of various sensors is reserved for the utilization of helicenes in chiral plasmonics. Unlike common detection methods, Surface-enhanced Raman Spectroscopy (SERS) represents an extremely sensitive method for the detection of substrates in concentrations as low as 10-13 M. The sensor is constructed by sorption, or chemical attachment of enantiomerically pure helicene molecule onto a nanostructured metal substrate, most often made of gold or silver. This setup allows for an interaction between the molecule of the chiral analyte and of the helicene, which in turn causes a change in the Raman response of the system.
These systems can be extensively tuned for various applications and analytes, by changing the structure and electronic properties, by variation of the contact between the metal surface and helicene, and also by engineering the surface of the metal. Our goal is to be able to distinguish between enantiomers of complex chiral compounds, especially those of biological relevance.
 |
 |
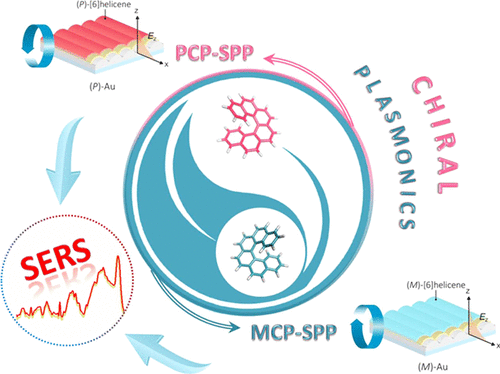 |
- Helicene-SPP-Based Chiral Plasmonic Hybrid Structure. ACS Appl. Mater. Interfaces 11, 1555–1562, 2019. DOI
- Chiroplasmon-active optical fiber probe. Sens. Actuators B Chem. 343, 130122, 2021. DOI
- Chiral Plasmonic Response of 2D Ti3C2Tx Flakes. Adv. Funct. Mater. 2023, 2212786, 2023. DOI
- Chiral nanoparticles with chiral helicene. Sens. Actuators B Chem. 394, 134332, 2023. DOI
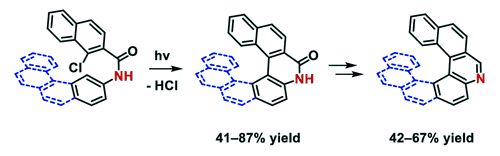
Preparation of new phenacene derivatives
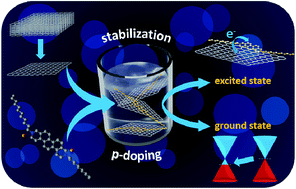
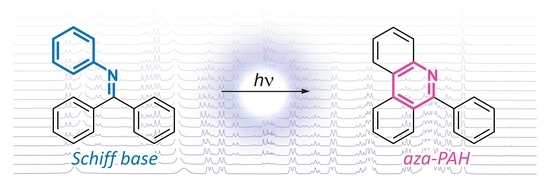
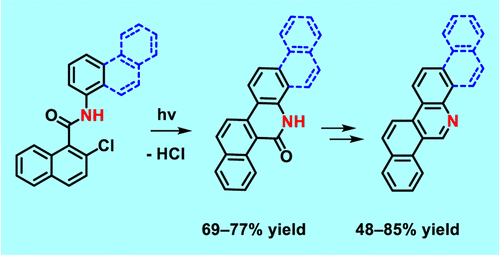
Phenacenes are known as stable compounds with resistance to oxygen and light, which makes these planar π-conjugated compounds suitable for utilization as functional materials in numerous applications. The introduction of nitrogen atoms into the phenacene skeleton leads to the formation of a dipole moment which is reflected in modified physicochemical properties when compared to their carbo analogs. Recently, a novel methodology for the synthesis of aza[n]phenacenes (n = 4 – 6) was successfully developed utilizing photocyclodehydrochlorination reaction of starting 2-chloro-N-aryl-1-naphthamides. Also, a novel N,N-didodecyl-3,10-diazapicenium salts with bromide and hexafluorophosphate counterions were synthesized as an exfoliant, a stabilizer, and a p-dopant for liquid phase exfoliated graphene.
 |
 |
 |
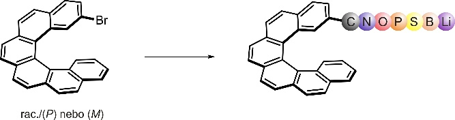
Preparation of new helicene derivatives
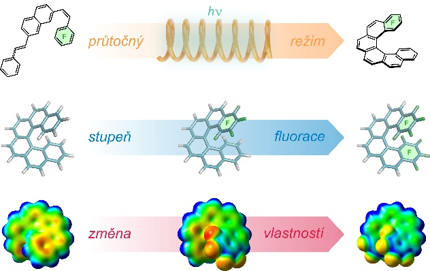
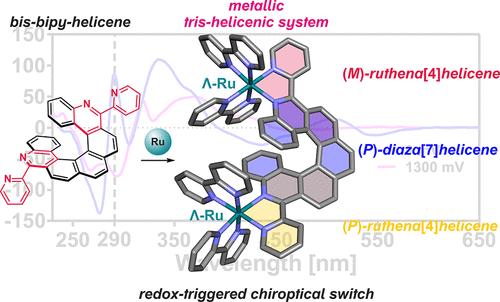
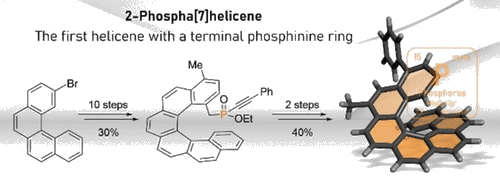
The synthetic approach leading to helicenes is based on a patented photooxidative cyclization of stilbene precursors. By transferring the synthesis to a flow system, we are capable of preparing helicenes on a multigram scale, which is sufficient not only for studying their properties and applications but also for further chemical transformations. Helicenes usually undergo reactions that are typical for simpler aromatics; their reactivity, however, is non-trivial, and thorough optimization is often required even for otherwise simple reactions. Nevertheless, it is often possible to introduce reactive functionalities either before the cyclization or in the later stages. This leads to the preparation of versatile materials with enormous application potential. Some helicene derivatives exhibit intrinsic photochemistry, which can be easily exploited for otherwise forbidden transformations.
 |
 |
|
|
- 9-Bromo[7]Helicene Reactivity. Tetrahedron 69(30), 6213–6218, 2013. DOI
- Helicene-Based Imidazolium Salt and Its Application in Organic Molecular Electronics. Chem. Eur. J. 21(6), 2343–2347, 2015. DOI
- Cation-π Interaction of Ag+ + [6]helicene. Chem. Phys. Lett. 633, 105-108, 2015. DOI
- Cation-π Interaction of Tl+ + [6]helicene. J. Mol. Struct. 1100, 150-153, 2015. DOI
- Chiral Resolution of Helicenes. J. Chromatogr. A 1476, 130-134, 2016. DOI
- Internal Dynamics in Helical Molecules. Phys. Chem. Chem. Phys. 19(4), 2900-2907, 2017. DOI
- 2-Bromo[6]Helicene Reactivity. J. Org. Chem. 83(7), 3607–3616, 2018. DOI
- [6]Helicenes Fluorinated at Terminal Rings. J. Org. Chem. 84(4), 1980–1993, 2019. DOI
- Cytotoxicity of Hexahelicene. Toxicology in Vitro 57, 105-109, 2019. DOI
- Photochemical Functionalization of Helicenes. Chem. Eur. J. 26(2), 543–547, 2020. DOI
- Synthesis of a Helical Phosphine. ACS Omega 5(1), 882-892, 2020. DOI
- Photochemical Oxidation of Helical Aromatic Amines. Org. Lett. 22(10), 3905-3910, 2020. DOI
- Recent Advances in Functionalizations of Helicene Backbone. J. Org. Chem. 85(11), 13415-13428, 2020. DOI
- Oxidative Photocyclization of Aromatic Schiff Bases. Inter. J. Mol. Sci. 21(16), 5868, 2020. DOI
- Enantioenriched Ruthenium Complexes of Azahelicenes. Inorg. Chem. 60(16), 11838-11851, 2021. DOI
- Flavin-Helicene Amphiphilic Hybrids. ChemPlusChem 86, 982, 2021. DOI
- Photochemical Approach to Helicenes. in book Helicenes: Synthesis, Properties, Applications 1-52, 2022. DOI
- Synthesis of Aza[n]helicenes (n = 4-7). J. Org. Chem. 87(11), 7150–7166, 2022. DOI
- Synthesis of 2-Phospha[7]helicene. Org. Lett. 24(26), 4756–4761, 2022. DOI
- Push-pull Phosphine Oxide-based Helicenes. Dyes Pig. 210, 111039, 2023. DOI
- Hexahelicene DNA-binding. Int. J. Biol. Macromol. 250, 125905, 2023. DOI
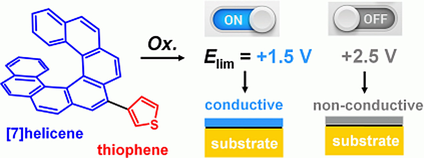
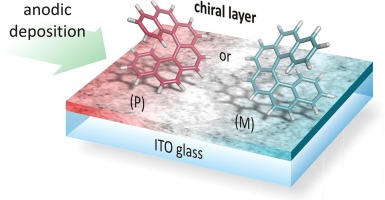
Electropolymerization of helicenes
Electropolymerization represents a unique approach to the fabrication of helicene-based thin layers on conductive substrates (ITO glass/foil, glassy carbon). Based on the used conditions it is possible to prepare both conductive and non-conductive films of various widths (from 10 nm). When an enantiomerically pure helicene is used, the layer retains its optical activity. This approach to thin layer fabrication can be advantageously employed in the preparation of optoelectronic devices and active parts of sensors.
 |
 |
 |
- Immobilization of Helicene onto Carbon Substrates. RSC Adv. 4(86), 46102–46105, 2014. DOI
- Electrosynthesis of [7]Helicene-Derived Polymers. ChemElectroChem 4(12), 3047–3052, 2017. DOI
- Anodic Deposition of Enantiopure [6]Helicene Layers. ChemElectroChem 5(15), 2080–2088, 2018. DOI
- Chiral Electrochemistry of Helical Molecules. ChemPlusChem 85(9), 1954-1958, 2020. DOI
- Carbohelicene layers prepared by potentiodynamic electropolymerization. Electrochem. Commun. 113, 10689, 2020. DOI
Commercialization of helicenes and cannabinoids
The development of multigram photochemical synthesis of helicenes and phenacenes was capitalized on in cooperation with Lach-Ner. Some common derivatives of helicenes have been available through their website since 2013. https://www.lach-ner.cz/heliceny
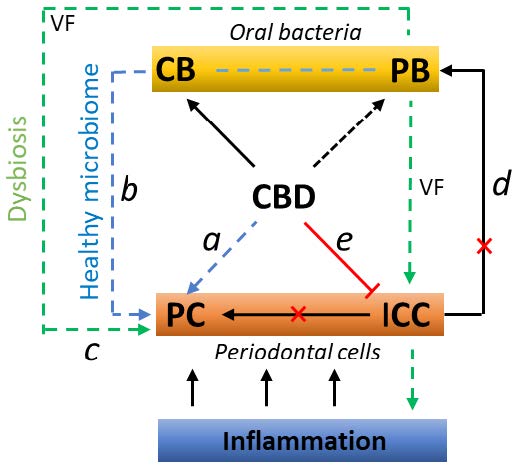
Between 2014 and 2016, our team contributed significantly to the development of extraction methods for the isolation of cannabinoids from varieties of industrial hemp. The developed know-how has been licensed to cooperating commercial partners who use it to produce cannabinoids.
 |
 |
 |
- CBD is converted to THC in rats. Eur. Neuropsychopharmacol. 50, 135-136, 2021. DOI
- The antioxidant function of phytocannabinoids. Free Radic. Biol. Med. 164, 258-270, 2021. DOI
- Cannabidiol and periodontitis. Biomedical Papers-Olomouc 166, 155-160, 2022. DOI
- Exposure to cannabidiol and cannabigerol. Toxicology 2023. DOI
- Hepatic biotransformation of non-psychotropic phytocannabinoids. Toxicol. Appl. Pharmacol. 476, 116654, 2023. DOI