Helical polyaromatic compounds, helicenes, attract attention due to their extraordinary optoelectronic properties, which predestine them for applications in the field of materials science. The ideal properties, however, are seldom found in unsubstituted carbohelicenes, and therefore it is necessary their further modification. In this context, scientists from the group of Dr. Storch developed a method by which an unusual phosphinine (phosphabenzene) ring can be introduced into the helicene backbone. The synthesis, characterization, and properties of this helicene were then published in the prestigious journal Organic Letters.
The 4-phenyl-6-methyl-2-phospha[7]helicene was prepared from starting 2‑bromobenzo[c]phenanthrene in 12% overall yield in 12 steps. In the described synthesis, the appropriate helicene precursor bearing an alkyne function is first prepared. The key step of the developed synthetic strategy is the acid-initiated cyclization of this alkyne yielding an ortho-fused phosphorus heterocycle, which is subsequently aromatized to form the target molecule. The structure of the first phosphahelicene with a terminal phosphinine ring was confirmed by X-ray crystallography.
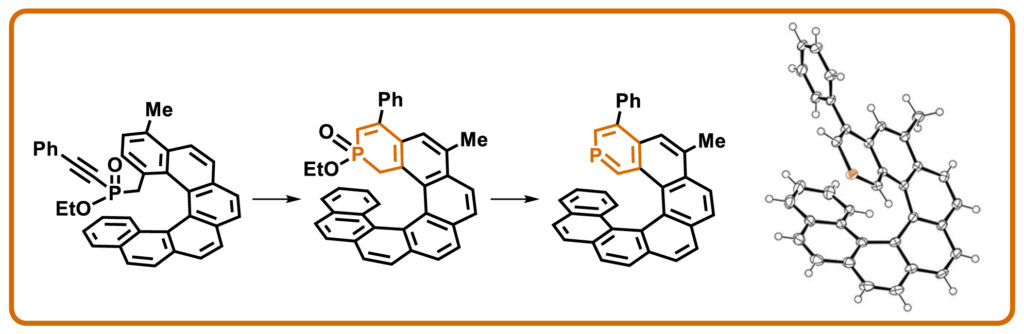
Synthesis of 4-phenyl-6-methyl-2-phospha[7]helicene
- Beránek, T.; Jakubec, M.; Sýkora, J.; Císařová, I.; Žádný, J.; Storch, J.: Synthesis of 2-Phospha[7]helicene, a Helicene with a Terminal Phosphinine Ring. Org. Lett. 2022, 24(26), 4756–4761. https://doi.org/10.1021/acs.orglett.2c01723