Bubble interactions and their coalescence is the topic studied for a long time at our Institute in Department of Multiphase Reactors. The research is focused on the fundamental study of bubble interaction mechanisms, their breakup and coalescence. These phenomena determine the bubble size distribution in the reactor, and consequently the interfacial area available for gas-liquid mass transfer. Current research was led by Dr. Orvalho and Doc. Růžička published recently in prestigious scientific journal, Chemical Engineering Journal, was focused on the effect of electrolytes on bubble coalescence process. Original experiments designed at our Institute enable to control the contact between pairs of bubbles in a liquid.
The research was focused on three characteristic variables: coalescence efficiency (i) describing the amount of coalescing bubbles from the total number of produced bubbles, transition electrolyte concentration (ii) when the system switches from coalescent to noncoalescent regime, and contact time (iii) measuring the time necessary till bubbles either coalesce or detach. It was shown that these characteristics do not depend only on the electrolyte type but depend also on the hydrodynamic conditions. Surprising and not known result is that bubble approach velocity plays a dual role in the coalescence process. On one hand, it reduces the coalescence region by shifting the transition electrolyte concentration to lower values and reducing the coalescence efficiency. On the other hand, it also accelerates the coalescence process by reducing the contact time. All tested electrolytes behave in a similar way and the effect of inorganic salts on the coalescence is similar to the effect of liquid viscosity, which was investigated at our Institute previously.
Orvalho, S., Stanovský, P., Růžička, M. Bubble coalescence in electrolytes: effect of bubble approach velocity. Chemical Engineering Journal. 2020, 125926. DOI: 10.1016/j.cej.2020.125926
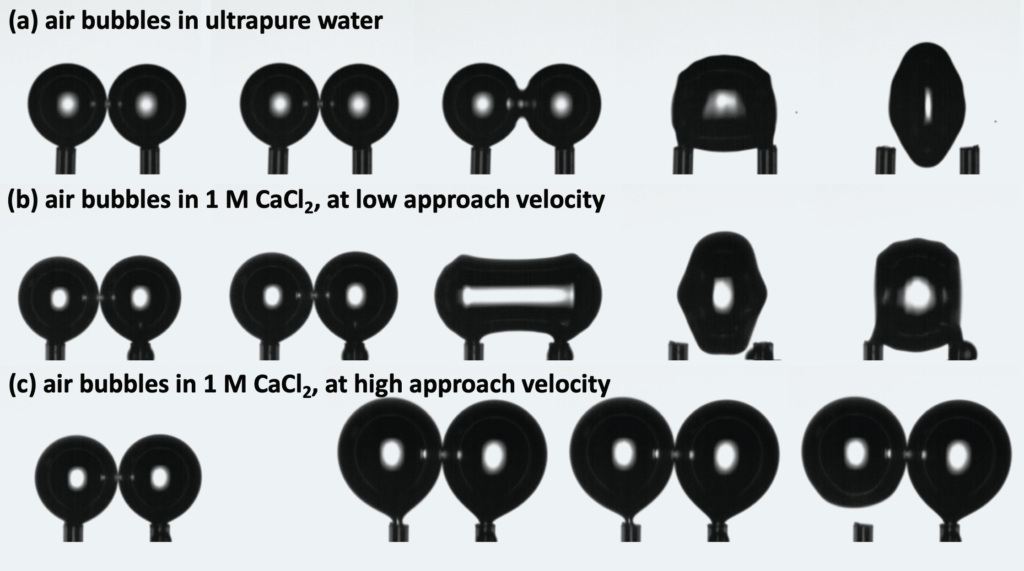
Example of bubble interaction and their coalescence: (a) air bubbles in ultrapure water – very fast coalescence; (b) air bubbles in 1M CaCl2 solution at low approach velocity – fast coalescence; (c) air bubbles in 1M CaCl2 solution at high approach velocity – no coalescence.