Carbosilane glycodendritic compounds designed for bio-applications
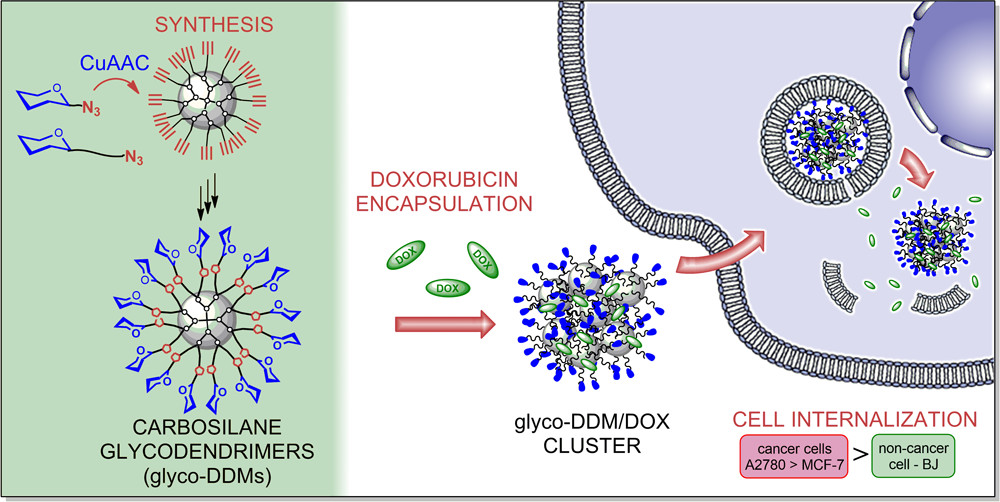
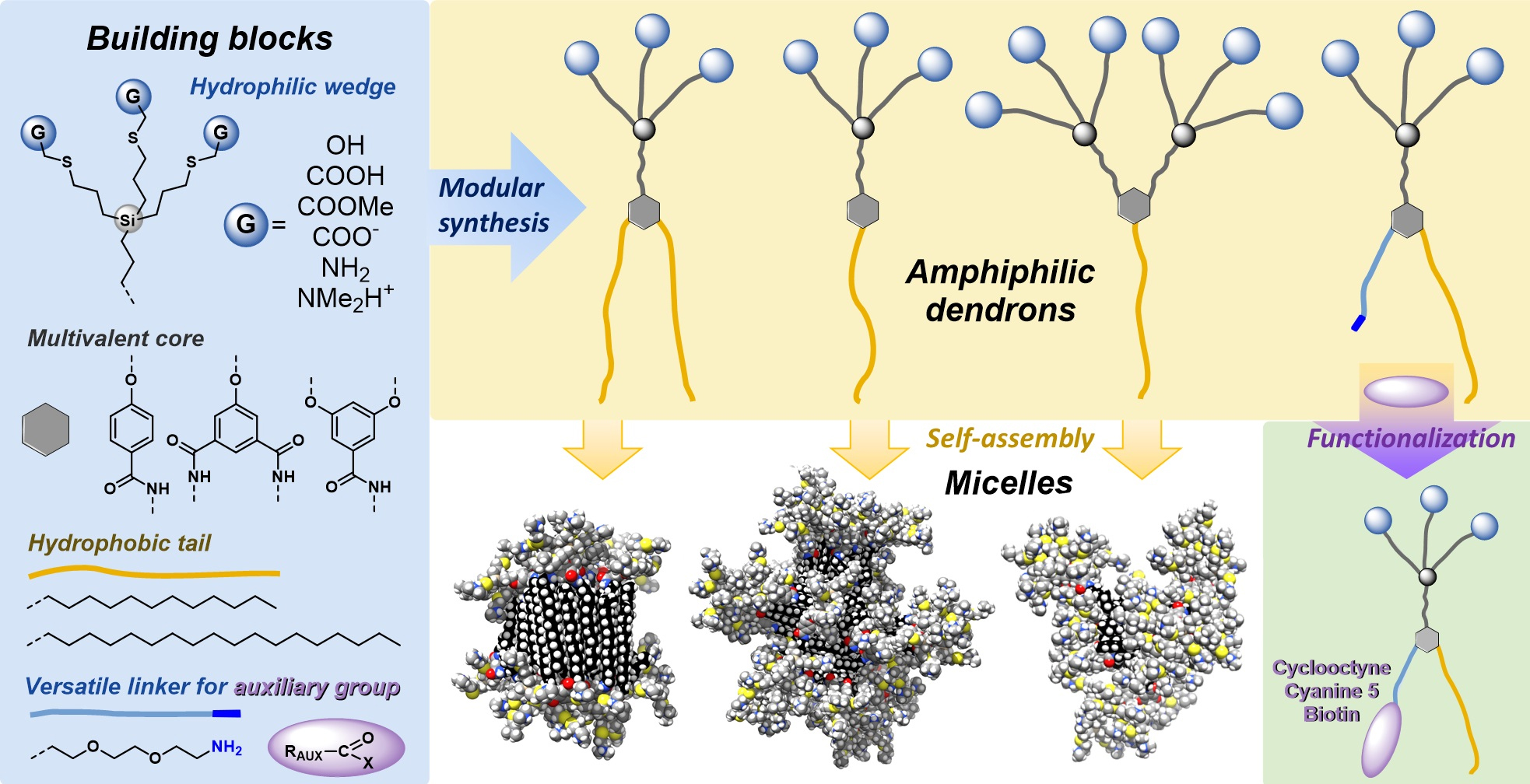
Carbosilane-based structures have advantageous properties for biomedically oriented research, such as high flexibility and stability and low cytotoxicity. We prepare dendritic platforms of different architectures with the silicon atom as the branching point. Our structurally well-defined libraries of dendrimers and dendrons are being systematically studied in various research directions. Dendrimers functionalized by galactose and glucose units were applied as highly biocompatible drug delivery systems for anticancer drug doxorubicin with high loading capacity, pH-dependent release, and preferential internalization in cancer cells. Modified fish embryo test (FET) was used for glucose dendrimers to investigate developmental toxicity on zebrafish (Danio rerio) embryos showing two to three orders of magnitude difference between the in vitrocytotoxicity and in vivo developmental toxicity. Recently prepared lactose-modified dendrimers with triazole ring in anomeric position are selective to sugar-binding protein galectin-9, exhibiting a huge positive dendritic effect compared to monovalent lactose. For gene delivery, we designed cationic lactose dendrimers with up to 32 charged units at the periphery. Besides their exceptional biocompatibility, the compounds formed stable complexes with siRNA. The modular synthetic approach allows us to design the conjugates rationally and to attach diverse functional, diagnostic, and bioactive moieties according to specific interests (fluorescent labels, reactive groups, short proteins, etc.).
 |
 |
- M. Müllerová, D. Maciel, N. Nunes, D. Wrobel, M. Stofik, L. Červenková Šťastná, A. Krupková, P. Cuřínová, K. Nováková, M. Božík, M. Malý, J. Malý, J. Rodrigues, T. Strašák: Biomacromolecules 23(1), 276–290, 2022. DOI
- A. Edr, D. Wrobel, A. Krupková, L. Červenková Šťastná, P. Cuřínová, A. Novák, J. Malý, J. Kalasová, J. Malý, M. Malý, T. Strašák: Int. J. Mol. Sci. 23(4), 2114, 2022. DOI
- D. Wrobel, M. Müllerová, T. Strašák, K. Růžička, M. Fulem, R. Kubíková, M. Bryszewska, B. Klajnert-Maculewicz, J. Malý: Int. J. Pharm. 579, 119138, 2020. DOI
- Nanotoxicology 12(8), 797–818, 2018. DOI
- Müllerová, M., Hovorková, M., Závodná, T., Červenková Šťastná, L., Krupková, L., Hamala, V., Nováková, K., Topinka, J., Bojarová, P., Strašák, T., Lactose-Functionalized Carbosilane Glycodendrimers Are Highly Potent Multivalent Ligands for Galectin-9 Binding: Increased Glycan Affinity to Galectins Correlates with Aggregation Behavior. Biomacromolecules 2023, 24 (11), 4705–4717. DOI: 10.1021/acs.biomac.3c00426