Receptors for anion complexation and chiral recognition

The receptors based on the axially chiral structure of binaphthyldiamine were designed and synthesized for interaction with alcohols, aldehydes, ketones or carboxylic acids. In the pilot study, the interactions of prepared receptors with chiral alcohols were thoroughly described. The structure of resulting diastereomeric complexes was proposed from NMR experiments and subsequently confirmed by DFT calculations. In the following study, the influence of ureido moiety substitution on the complexation properties of the receptor was tested. Arylpropanoic acids, known as profens in medicinal chemistry, were used as substrates and served for determination of detection limits for each enantiomer. Binaphthyldiamine-based ureido receptors were recently applied for determination of the absolute configuration of ethyl 4-chloro-3-hydroxybutyrate prepared in the microfluidic reactor via enantioselective reaction. A receptor anchoring on various carrier structures is currently in progress.
Interaction of receptors with chiral alcohols, Chirality 2018, 30, 798–806. DOI:10.1002/chir.22855
Complexation of profens, Chirality 2019, 31, 410– 417. DOI:10.1002/chir.23067
4-chloro-3-hydroxybutyrate, J Flow Chem 2019. DOI:10.1007/s41981-019-00043-y
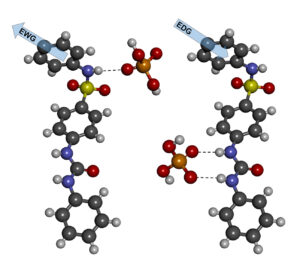 As a part of work at our GAČR supported project New Nanomaterials for Anion Separation, a study was performed concerning the newly prepared receptor moieties targeted towards complexation of phosphate. During this detailed examination we observed the influence of substituents attached to basic structure of a receptor on the behaviour of whole receptor molecule in the presence of phosphate anion. To increase the receptor effectiveness, the electrons were withdrawn from urea-based complexation site by a sulphonamidic group. We proved, that a suitable chemical modification of electron environment near the sulphonamidic group can protect this site of unwanted deprotonation. On the other hand, the sulphonamidic group acidity can be utilised for attachment of the final receptor moiety to a suitable carrier.
As a part of work at our GAČR supported project New Nanomaterials for Anion Separation, a study was performed concerning the newly prepared receptor moieties targeted towards complexation of phosphate. During this detailed examination we observed the influence of substituents attached to basic structure of a receptor on the behaviour of whole receptor molecule in the presence of phosphate anion. To increase the receptor effectiveness, the electrons were withdrawn from urea-based complexation site by a sulphonamidic group. We proved, that a suitable chemical modification of electron environment near the sulphonamidic group can protect this site of unwanted deprotonation. On the other hand, the sulphonamidic group acidity can be utilised for attachment of the final receptor moiety to a suitable carrier.
ChemPlusChem 2020. DOI:10.1002/cplu.202000326