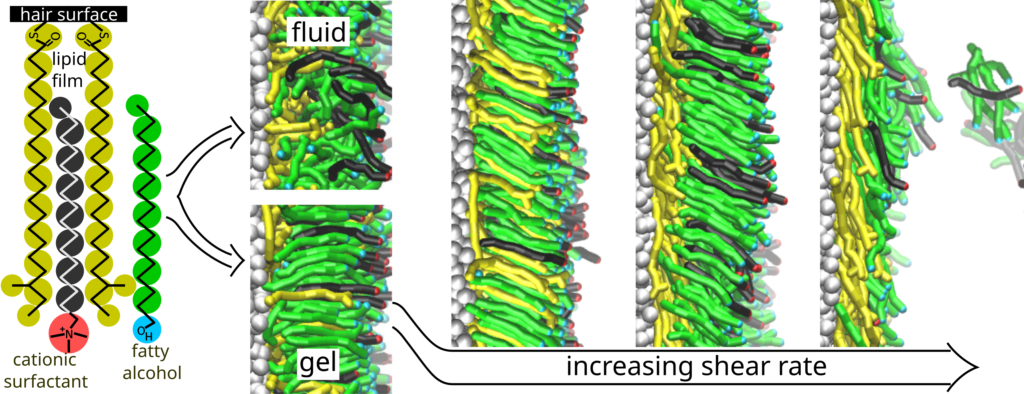
Interactions of cationic surfactant-fatty alcohol monolayers with natural human hair surface: Insights from dissipative particle dynamics
Fatty alcohols (CnFAs) combined with cationic surfactants are common ingredients of lamellar-phase personal care liquids. We employ mesoscopic modelling to study how surfactant-CnFA monolayers originating from the corresponding bilayers of the personal care liquids interact with the human hair surface above and below the fluid-gel transition temperature as well as in- and out-of-equilibrium. For the monolayer model, we consider the single-tail cationic surfactant cetyltrimethylammonium chloride (CTAC) and an excess of CnFAs with their alkyl tail length equal to or longer than the CTAC alkyl tail length.
The hair surface mimics keratin surface proteins covered by a film of lipid chains covalently bonded to the proteins. Our modelling shows the formation of a dense adsorbed layer due to the interactions of the CTAC and CnFA alkyl tails with the hydrophobic hair surface. The adsorption and the behaviour of the adsorbed layer is different under fluid and gel conditions. The differences are related to the structure of the adsorbed layer as characterised by density profiles across the adsorbed layer and the orientational order parameters of the chains within the adsorbed layer. Under steady-state shearing (an approximation of real, non-equilibrium conditions), increasing the shear rate above a threshold leads to continuous or abrupt desorption of the CTAC and CnFA chains under fluid or gel conditions, respectively; the desorbed chains can then form various self-assembled structures in the bulk solution. The underlying mechanism of CTAC and CnFA desorption from the adsorbed layers is closely related to the corresponding adsorption mechanism.
- Karel Šindelka, Adam Kowalski, Michael Cooke, César Mendoza, Martin Lísal*: Interactions of cationic surfactant-fatty alcohol monolayers with natural human hair surface: Insights from dissipative particle dynamics. Journal of Molecular Liquids 2023, 375, 121385. doi: 10.1016/j.molliq.2023.121385.