Fluorinated carbohydrates
Fluorination is an extremely useful tool to probe and modulate carbohydrate-protein interactions. We focus on the synthesis of fluorocarbohydrates, especially fluorinated oligosaccharides, and their use as glycomimetic probes of carbohydrate-protein interactions. Our particular interest is to employ fluorinated oligosaccharides as selective ligands for galectins, a biomedically significant class of carbohydrate-binding proteins. We are developing methods for the preparation of fluorinated glycosyl donors and acceptors, and studying their reactivity and stereoselectivity in glycosylation. Systematic regio- and stereoselective fluorination of each position within the sugar skeleton permits to site-selectively decouple each individual carbohydrate hydroxyl from the hydrogen-bonding network within the glycan chain and evaluate its influence on conformation, lipophilicity, and other properties. Similarly, systematic fluorination can reveal the contribution of each position within the oligosaccharide framework to glycan-protein interaction. In addition, new binding interactions can be introduced by fluorination in some cases. Taking advantage of the favorable NMR properties of the 19F nucleus, we can investigate carbohydrate-protein interactions through 19F NMR-based experiments.
Carbohydrate – organometallic conjugates
Development and application of multivalent dendritic platforms
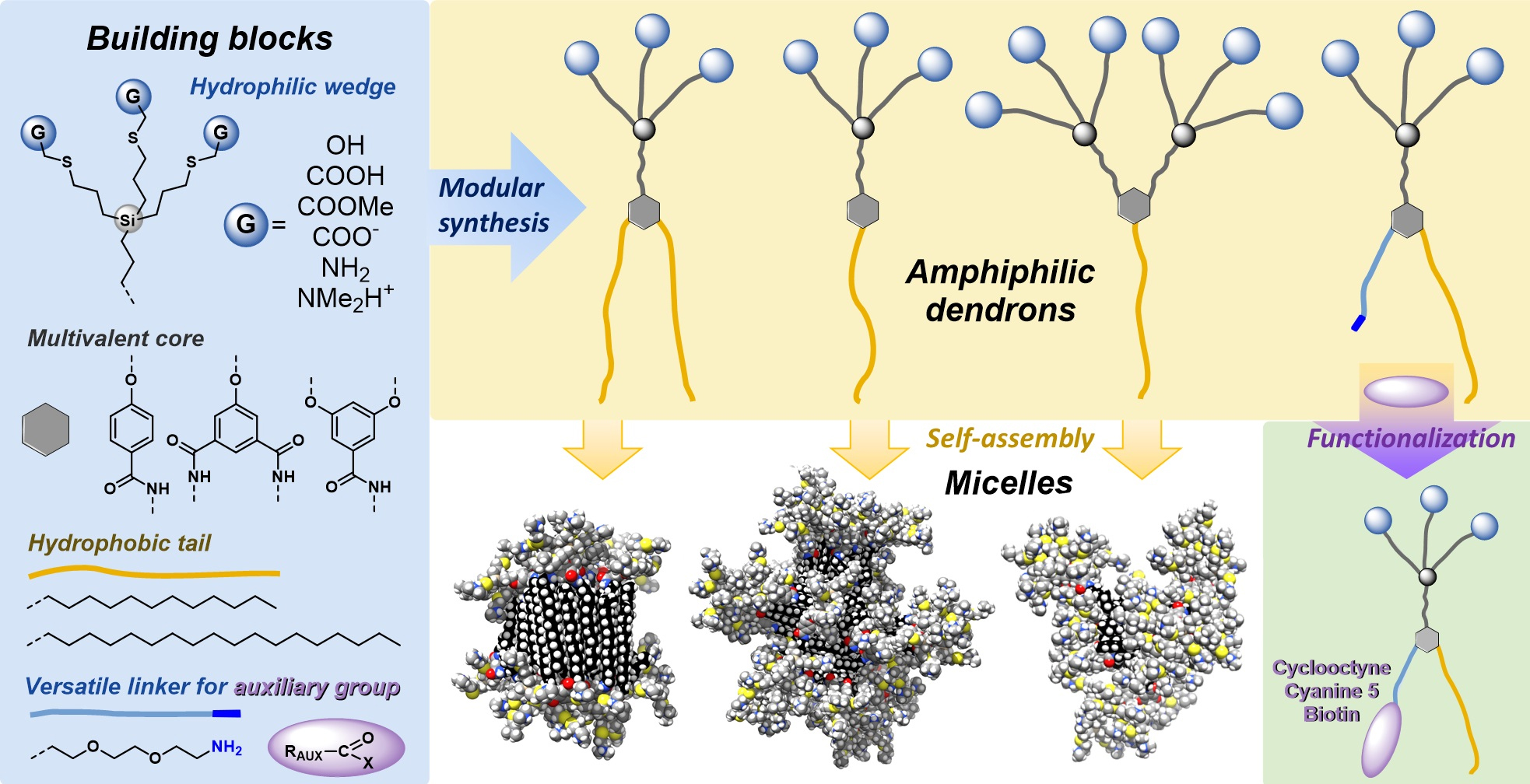
In our group, we develop new concepts and the respective synthetic and separation methods for the preparation and isolation of carbosilane dendrimers and complex macromolecular and supramolecular systems based on dendritic architectures. The outer shell of the dendrimer (periphery) contains multiple well-defined anchoring points, suitable for attachment of functional moieties targeted to particular applications. The modular synthetic approach allows us to design specific heterofunctional materials. We focus especially on the synthesis of multivalent glycoconjugates and anion receptors.
Multivalent glycosylated materials play an important role in glycobiology, e.g., as enzyme inhibitors or model clustered systems to study interactions between saccharides and lectins (sugar-binding proteins), or to use them as nonviral vectors and drug delivery systems. At present, we develop multivalent dendritic systems presenting not only natural ligands of lectins and carbohydrate transporters, i.e. glucose, galactose, lactose, and N-acetyl-d-lactosamine but also their synthetic analogs and derivatives.
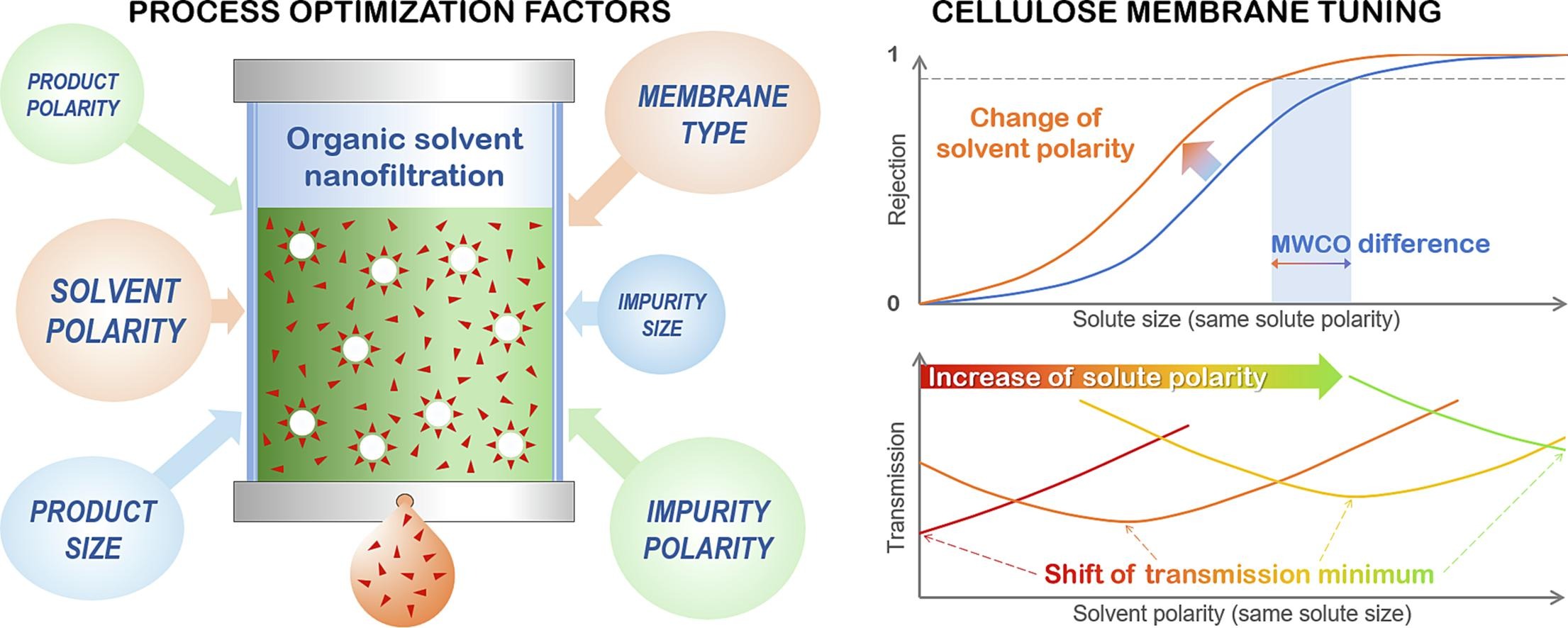
In addition, we are interested in the preparation of recyclable receptors of biologically important anions (H2PO4-, carboxylates, chiral carboxylic acids, etc.). Active sites recognizing particular analytes are anchored to a dendritic carbosilane carrier, which, besides multiplying the binding sites, enables their cooperation on analyte coordination. Moreover, such receptors can be used to separate the analyte from a mixture in the form of a macromolecular complex. Induced dissociation of a complex and nanofiltration leads to receptor recycling and to the separation of the targeted analyte.
Characterization and purification of carbosilane dendritic compounds
The synthesis of dendritic molecules is inherently connected with the development of innovative purification and characterization methods. We are interested in detailed analysis of dendritic structure and we were able to elucidate the structure and origin of most defects accompanying the synthesis of carbosilane dendrimers as well as their peripheral functionalization. Especially in soft ionization mass spectrometry (ESI, MALDI), the high symmetry and repetitive structure of dendrimers have an amplification effect and lead to higher sensitivity of the method to defects. This information is important for further optimization of synthetic protocols in order to obtain high-purity products suitable for bioorganic applications. Implementation of nanofiltration techniques, both in the aqueous phase and in organic solvents, enabled us to further increase the purity of our products and also to recycle valuable reagents that have to be usually applied in excess.
- A. Krupková, M. Müllerová, R. Petrickovic, T. Strašák: Sep. Purif. Technol. 310, 123141, 2023. DOI
- P. Cuřínová, A. Krupková, L. Červenková Šťastná, M. Müllerová, J. Čermák, T. Strašák: J. Mass Spectrom. 53, 986–996, 2018. DOI
- A. Krupková, J. Čermák, Z. Walterová, J. Horský: Macromolecules 43, 4511–4519, 2010. DOI
- A. Krupková, J. Čermák, Z. Walterová, J. Horský: Anal. Chem. 79, 1639-1645, 2007. DOI
Carbosilane glycodendritic compounds designed for bio-applications
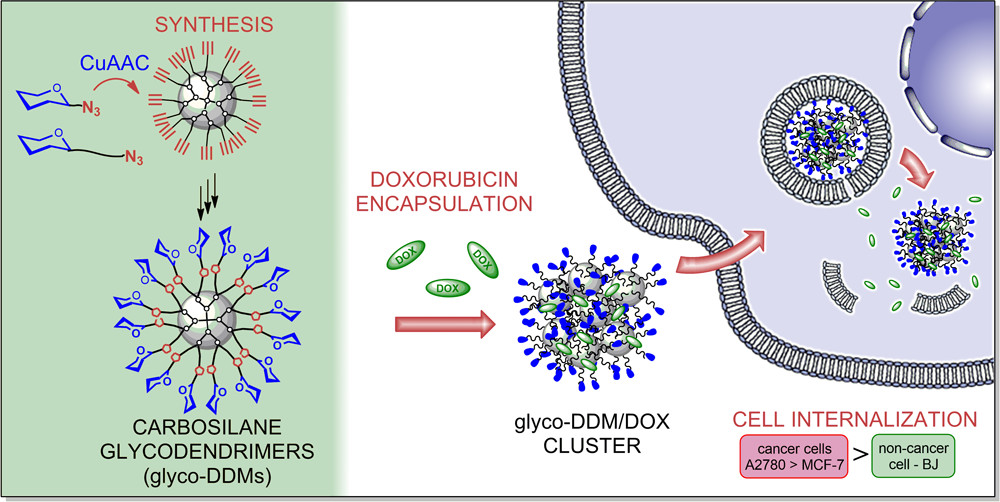
Carbosilane-based structures have advantageous properties for biomedically oriented research, such as high flexibility and stability and low cytotoxicity. We prepare dendritic platforms of different architectures with the silicon atom as the branching point. Our structurally well-defined libraries of dendrimers and dendrons are being systematically studied in various research directions. Dendrimers functionalized by galactose and glucose units were applied as highly biocompatible drug delivery systems for anticancer drug doxorubicin with high loading capacity, pH-dependent release, and preferential internalization in cancer cells. Modified fish embryo test (FET) was used for glucose dendrimers to investigate developmental toxicity on zebrafish (Danio rerio) embryos showing two to three orders of magnitude difference between the in vitrocytotoxicity and in vivo developmental toxicity. Recently prepared lactose-modified dendrimers with triazole ring in anomeric position are selective to sugar-binding protein galectin-9, exhibiting a huge positive dendritic effect compared to monovalent lactose. For gene delivery, we designed cationic lactose dendrimers with up to 32 charged units at the periphery. Besides their exceptional biocompatibility, the compounds formed stable complexes with siRNA. The modular synthetic approach allows us to design the conjugates rationally and to attach diverse functional, diagnostic, and bioactive moieties according to specific interests (fluorescent labels, reactive groups, short proteins, etc.).
 |
 |
- M. Müllerová, D. Maciel, N. Nunes, D. Wrobel, M. Stofik, L. Červenková Šťastná, A. Krupková, P. Cuřínová, K. Nováková, M. Božík, M. Malý, J. Malý, J. Rodrigues, T. Strašák: Biomacromolecules 23(1), 276–290, 2022. DOI
- A. Edr, D. Wrobel, A. Krupková, L. Červenková Šťastná, P. Cuřínová, A. Novák, J. Malý, J. Kalasová, J. Malý, M. Malý, T. Strašák: Int. J. Mol. Sci. 23(4), 2114, 2022. DOI
- D. Wrobel, M. Müllerová, T. Strašák, K. Růžička, M. Fulem, R. Kubíková, M. Bryszewska, B. Klajnert-Maculewicz, J. Malý: Int. J. Pharm. 579, 119138, 2020. DOI
- Nanotoxicology 12(8), 797–818, 2018. DOI
- Müllerová, M., Hovorková, M., Závodná, T., Červenková Šťastná, L., Krupková, L., Hamala, V., Nováková, K., Topinka, J., Bojarová, P., Strašák, T., Lactose-Functionalized Carbosilane Glycodendrimers Are Highly Potent Multivalent Ligands for Galectin-9 Binding: Increased Glycan Affinity to Galectins Correlates with Aggregation Behavior. Biomacromolecules 2023, 24 (11), 4705–4717. DOI: 10.1021/acs.biomac.3c00426
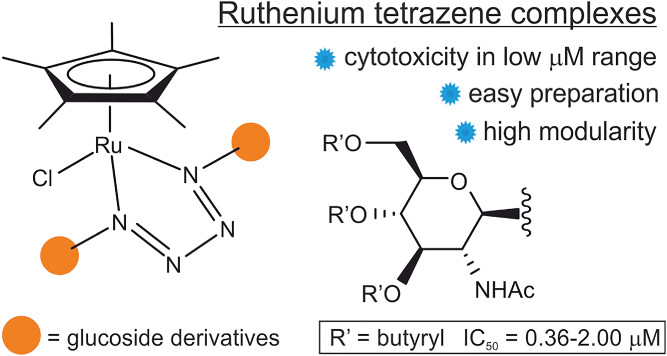
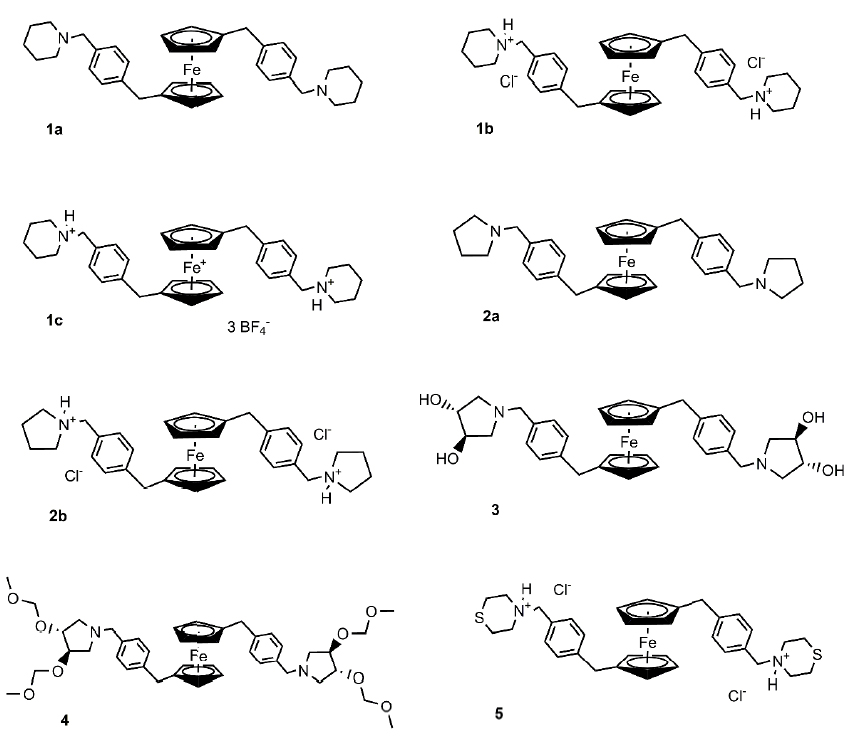
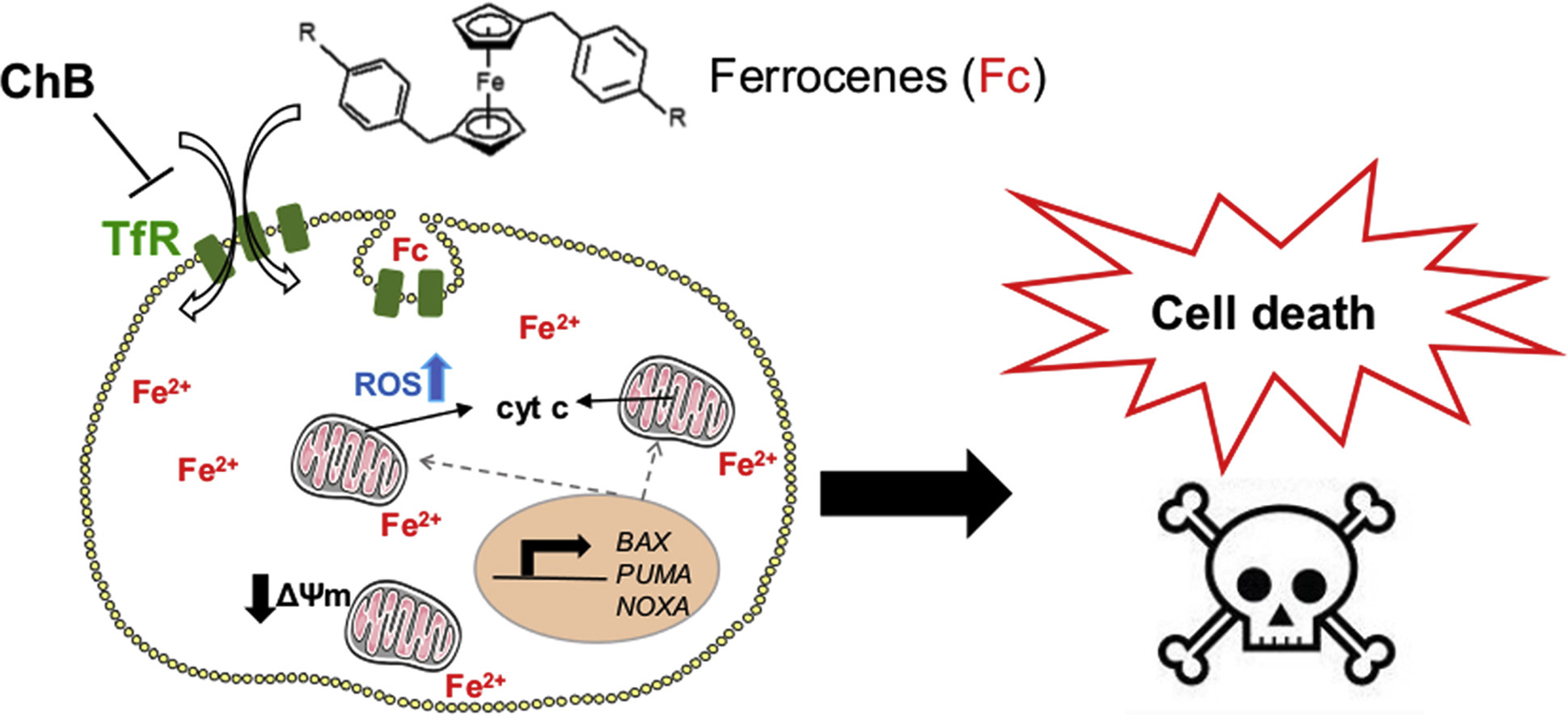
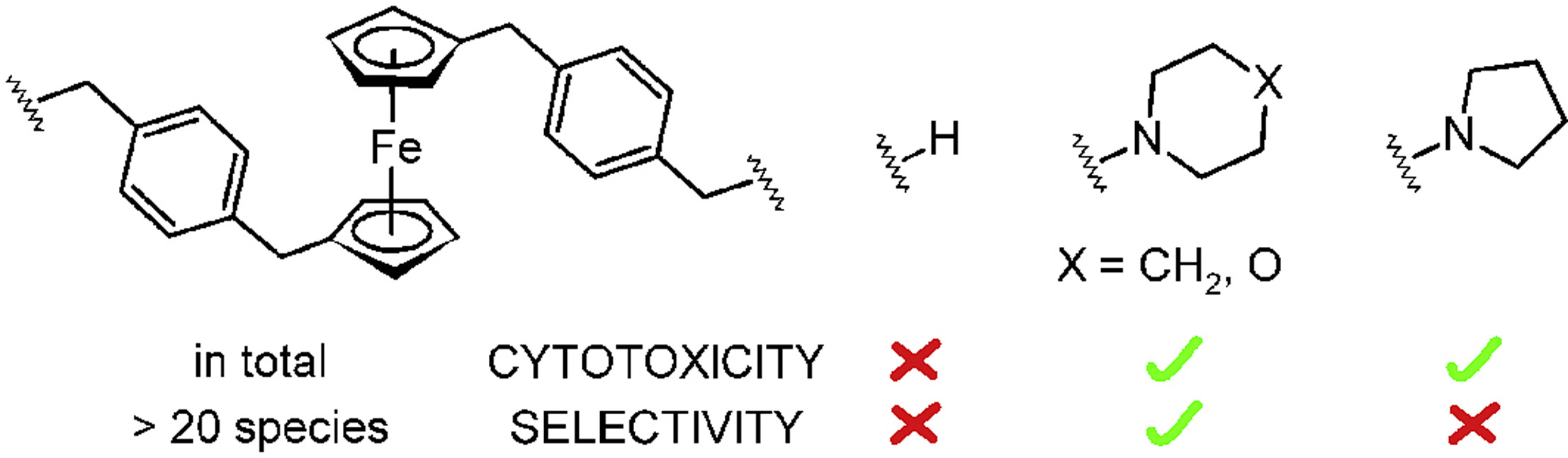
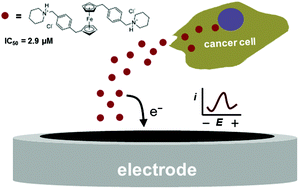
Antitumor organometallic glycoconjugates
Introduction of platinum complexes as chemotherapeutics in anticancer treatment initiated extensive research into the antitumor activity of organometallic compounds. We are interested in antitumor complexes containing a suitably modified carbohydrate moiety as a ligand complexed to the metal centrum. We found that some types of complexes, e.g. ruthenium tetrazenes or some ferrocene and titanocene analogs, are highly cytotoxic, whereas ruthenium arenes display considerable antimetastatic activity while being essentially non-cytotoxic.
 |
 |
 |
 |
 |
 |
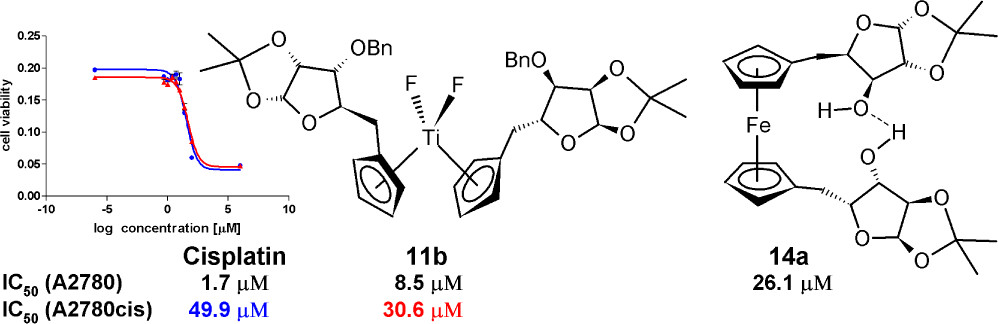 |
- Hamala, V.; Ondrášková, K.; Červenková Šťastná, L.; Krčil, A.; Müllerová, M.; Kurfiřt, M.; Hiršová, K.; Holčáková, J.; Gyepes, R.; Císařová, I.; Bernášková, J., Hrstka, R; Karban, J. Improving the anticancer activity of fluorinated glucosamine and galactosamine analogs by attachment of a ferrocene or ruthenium tetrazene motif. Appl. Organomet. Chem. 2024, 38 (5), e7399. DOI: 10.1002/aoc.7399.
- M. Lamač, M. Horáček, L. Červenková Šťastná, J. Karban, L. Sommerová, H. Skoupilová, R. Hrstka, J. Pinkas: Appl. Organomet. Chem. 34(1), e5318, 2020. DOI
- V. Hamala, A. Martišová, L. Červenková Šťastná, J. Karban, A. Dančo, A. Šimarek, M. Lamač, M. Horáček, T. Kolářová, R. Hrstka, R. Gyepes, J. Pinkas: Appl. Organomet. Chem. 34(11), e5896, 2020. DOI
- H. Skoupilova, V. Rak, J. Pinkas, J. Karban, R. Hrstka: Appl. Sci. 10(11), 3728, 2020. DOI
- H. Skoupilova, M. Bartosik, L. Sommerova, J. Pinkas, T. Vaculovic, V. Kanicky, J. Karban, R. Hrstka: Eur. J. Pharmacol. 867, 172825, 2020. DOI
- T. Hodík, M. Lamač, L. Červenková Šťastná, P. Cuřínová, J. Karban, H. Skoupilová, R. Hrstka, I. Císařová, R. Gyepes, J. Pinkas: J. Organomet. Chem. 846, 141–151, 2017. DOI
- M. Bartošík, L. Koubková, J. Karban, L. Červenková Šťastná, T. Hodík, M. Lamač, J. Pinkas, R. Hrstka: Analyst 140(17), 5864-5867, 2015. DOI
- T. Hodík, M. Lamač, L. Červenková Št’astná, J. Karban, L. Koubková, R. Hrstka, I. Císařová, J. Pinkas: Organometallics 33(8), 2059-2070, 2014. DOI
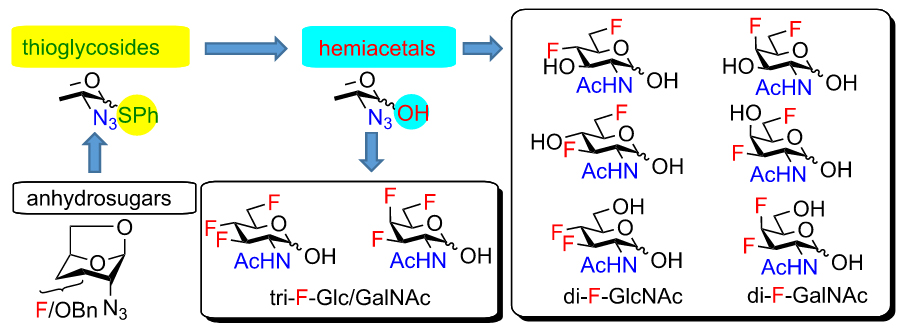
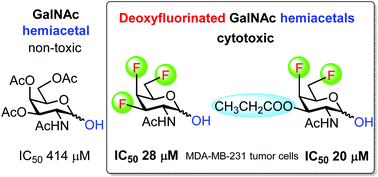
Synthesis and cytotoxicity of multiply fluorinated carbohydrates
We have prepared a complete series of mono-, di- and trifluorinated analogs of N-acetylglucosamine and N-acetylgalactosamine. Most of them were obtained using 1,6-anhydropyranose chemistry for stereoselective introduction of fluorine at the 3- and 4-positions. The application of fluorinated 2-azido-1-thioglycoside intermediates permitted chemoselective manipulation of the anomeric position. We used this approach for the preparation of fluorinated acylated 2-acetamido-lactols, which exhibited cytotoxicity in the concentration of low tens of micromole.
 |
 |
Stereoselectivity in glycosylation with fluorinated carbohydrates
The stereoselective formation of a glycosidic bond remains the main challenge associated with the chemical synthesis of complex glycostructures. The influence of substituents at remote positions of the pyranose ring on the stereoselectivity of glycosylation is increasingly recognized. We study how fluorine substituents at C3, C4, and C6 of 2-azido-hexopyranosyl donors affect the stereoselectivity in glycosylation. We use fluorinated thioglycoside donors prepared from our recently synthesized fluorinated 2-azido-1,6-anhydro-hexopyranoses. Their stereoselectivities are compared to those of the corresponding O-benzylated and O-acetylated analogs and the mechanistic interpretation is suggested. Using a modification of both glycosyl donors and acceptors and varying reaction conditions, we are able to develop procedures for 1,2-cis glycosylation with deoxofluorinated 2-azido-2-deoxy-d-gluco/galactopyranosyl donors.
 |
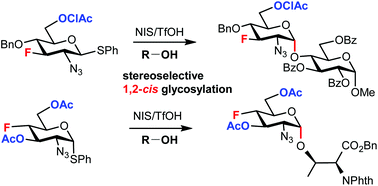 |
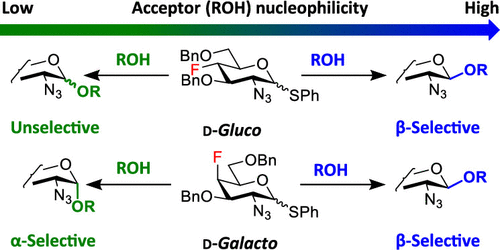 |
- M. Kurfiřt, L. Červenková Št’astná, P. Cuřínová, V. Hamala, J. Karban: J. Org. Chem. 86(7), 5073–5090, 2021. DOI
- V. Hamala, L. Červenková Št’astná, M. Kurfiřt, P. Cuřínová, M. Dračínský, J. Karban: Org. Biomol. Chem. 18(28), 5427–5434, 2020. DOI
- M. Kurfiřt, L. Červenková Št’astná, M. Dračínský, M. Müllerová, V. Hamala, P. Cuřínová, J. Karban: J. Org. Chem. 84(10), 6405–6431, 2019. DOI
The use of fluorinated oligosaccharides as probes for galectins
G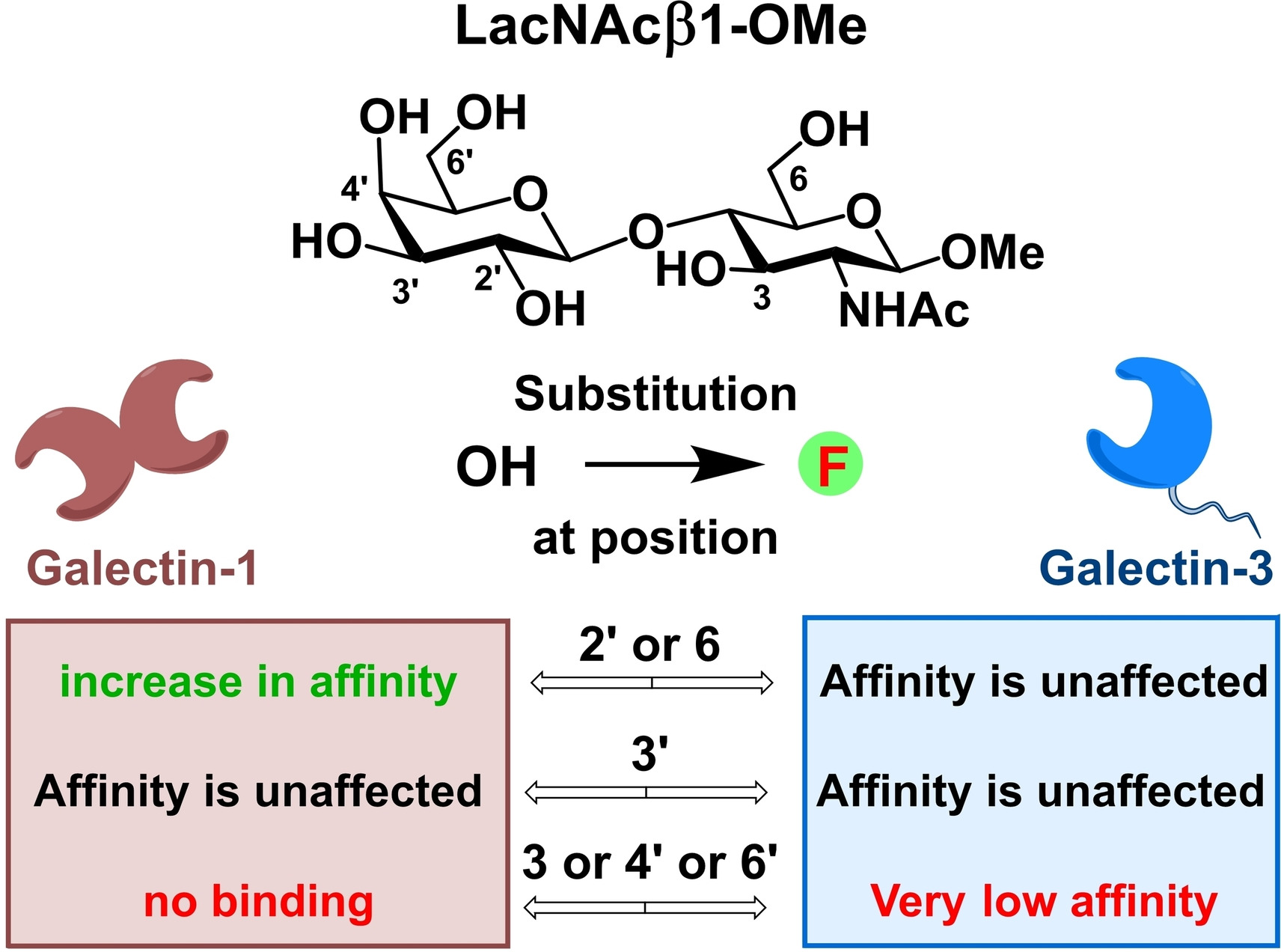 alectins are soluble proteins, which non-covalently bind β-galactosides. Non-covalent interactions of galectins with oligosaccharides containing this structural motif are implied in pathologies including cancer, inflammation, and fibrotic diseases. We synthesize fluorinated analogs of galectin ligands including disaccharides lactose, N-acetyllactosamine, and N,N’-diacetyllactosamine, and use them as probes for a panel of human galectins. In addition to the chemical mapping of the binding site, we also employ 19F NMR methods to elucidate the molecular basis of the observed binding phenomena. The attachment of monovalent fluorinated disaccharides to multivalent carriers (e.g. dendrimers and proteins) enables us to assess the impact of clustered presentation on affinity and selectivity. Modulation of the ligand selectivity to galectins by deoxyfluorination and ligand clustering is expected to lead to the identification of selective inhibitors. Fluorinated tetrasaccharides formed by β-(1-4) glycosidic attachment of selected fluorinated disaccharides to natural or fluorinated lactose or N-acetyllactosamine is currently employed to map the less conserved binding subsites A and B, whose binding preferences towards glycomimetics are mostly unknown.
alectins are soluble proteins, which non-covalently bind β-galactosides. Non-covalent interactions of galectins with oligosaccharides containing this structural motif are implied in pathologies including cancer, inflammation, and fibrotic diseases. We synthesize fluorinated analogs of galectin ligands including disaccharides lactose, N-acetyllactosamine, and N,N’-diacetyllactosamine, and use them as probes for a panel of human galectins. In addition to the chemical mapping of the binding site, we also employ 19F NMR methods to elucidate the molecular basis of the observed binding phenomena. The attachment of monovalent fluorinated disaccharides to multivalent carriers (e.g. dendrimers and proteins) enables us to assess the impact of clustered presentation on affinity and selectivity. Modulation of the ligand selectivity to galectins by deoxyfluorination and ligand clustering is expected to lead to the identification of selective inhibitors. Fluorinated tetrasaccharides formed by β-(1-4) glycosidic attachment of selected fluorinated disaccharides to natural or fluorinated lactose or N-acetyllactosamine is currently employed to map the less conserved binding subsites A and B, whose binding preferences towards glycomimetics are mostly unknown.
- M. Kurfiřt, M. Dračínský, L. Červenková Šťastná, P. Cuřínová, V. Hamala, M. Hovorková, P. Bojarová, J. Karban: Chem. Eur. J. 27(51), 13040–13051, 2021. DOI
Recent publications
|
2024 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
2022 |
|
|
|
|
|
|
|
|
|
2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2019 |
|
|
|
|
|