Preparing organophosphorus π-conjugated systems is one of the long-standing themes of the Research Group of Advanced Materials and Organic Synthesis (GAMOS). These systems have become the subject of intense study in recent years because the introduction of a phosphorus cycle into the polycyclic aromatic compounds not only increases the solubility of these substances in common organic solvents but also enhances photostability, provides ambipolar redox properties and often improves electroluminescence both in solution and in the solid state. The presence of the phosphorus atom then facilitates further derivatization (oxidation (O, S, Se), borylation, alkylation, metal complex formation), effectively modifying some key properties of the target molecules (HOMO/LUMO difference, charge transfer rate, triplet-triplet spin interaction, absorption and luminescence properties) and thereby tailoring them to the intended application. As a result, compounds containing the phosphorus cycle were already used as biomarkers for cancer therapy, as sensors and catalysts, or found applications in the material chemistry (OLED, EFD).
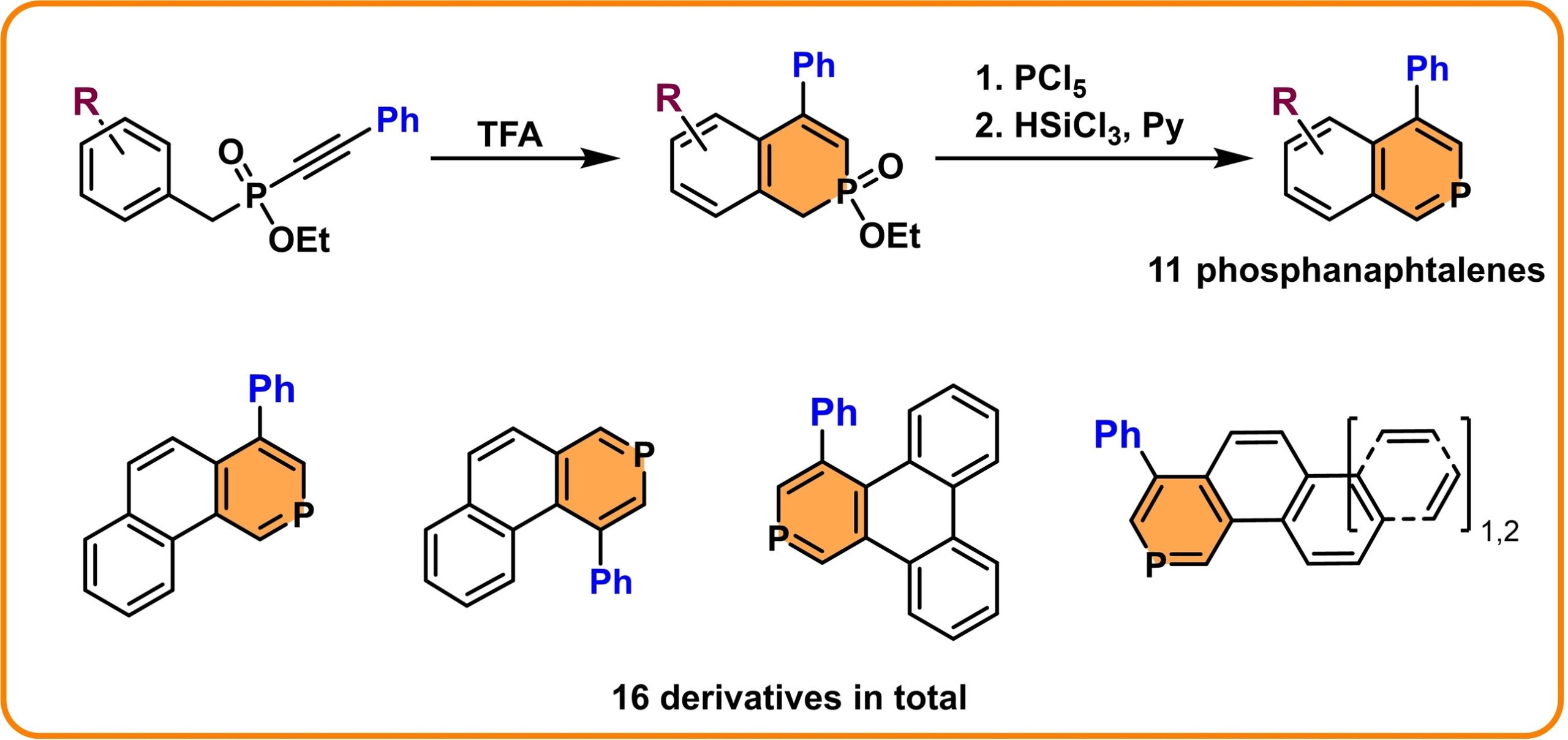
A recent paper published in Advanced Synthesis and Catalysis also falls into this area. The publication deals with preparing (poly)cyclic aromatic compounds based on phosphine. The preparation of these compounds has been rare and is often hampered by various limitations, such as the use of complex synthetic procedures or the narrow group of similar derivatives that could be obtained by the specific method. Now we have developed a robust and efficient synthesis that can introduce the phosphinine ring into aromatic compounds with relative simplicity. As a result, several phosphanaphthalene derivatives and, subsequently, several larger polycyclic aromatic systems containing phosphinine with potential applications in materials chemistry were prepared.
- Mizerová E., Kos M., Jakubec M., Pavlica M., Žádný J., Církva V., Storch J., Beránek T.*: Introduction of Phosphinine Ring into Aromatic Systems via Alkyne Cyclization. Advanced Synthesis & Catalysis 2025, 367, e202401203. doi.org/10.1002/adsc.202401203