Molecular simulations of cesium halide aqueous solutions and crystalline salts using phase-transferable polarizable force fields
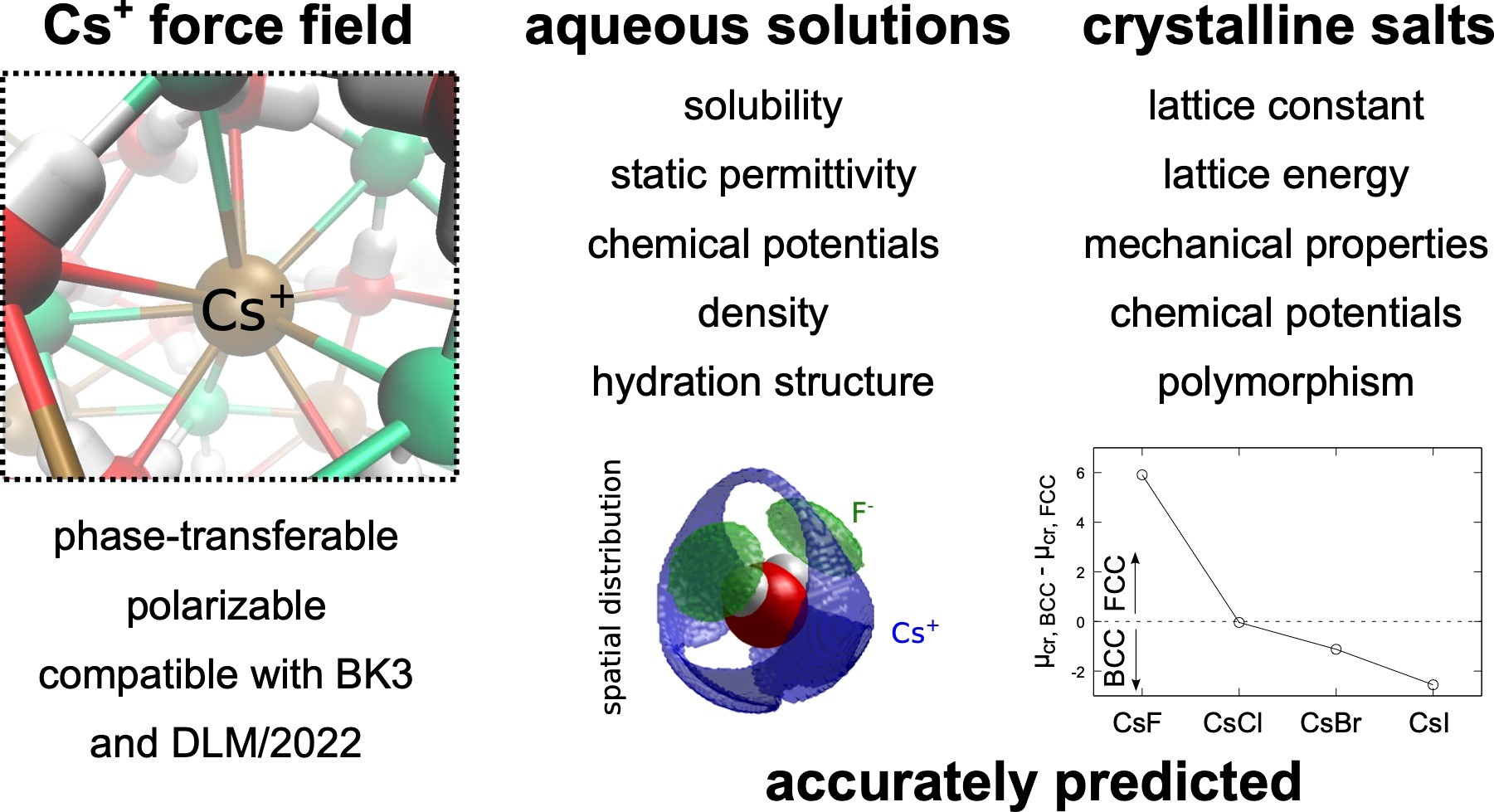
Cesium, an important alkali metal halide, exhibits distinct behavior necessitating accurate modeling of its interactions. We present a refined polarizable force field for Cs+ ions, extending the set of DLM/2022-BK3 FFs, to improve predictions of cesium halide properties. The refined model accurately captures structural properties, phase transitions, and concentration-dependent properties of aqueous solutions of CsF, CsCl, CsBr, and CsI, including high concentrations. Structural insights reveal variations in hydration numbers and the formation of contact ion pairs, especially pronounced with larger counterions. Notably, the force fields accurately predict aqueous solubilities, highlighting their accuracy in both ion-water and ion-ion interactions. While primarily designed for single salt solutions, these models offer broad applicability, extending to complex mixtures. Our findings underscore the reliability and versatility of the developed Cs+ model and the DLM/2022-BK3 force fields, which provides a valuable tool for studying alkali metal halide systems.
- J. Dočkal, P. Mimrová, M. Lísal, F. Moučka: Molecular simulations of cesium halide aqueous solutions and crystalline salts using phase-transferable polarizable force fields. J. Mol. Liq. 424, 127121, 2025. DOI