Understanding interfacial structure and preferential adsorption in mixed alkali-halide electrolytes at graphene oxide electrodes by constant potential molecular dynamics simulations
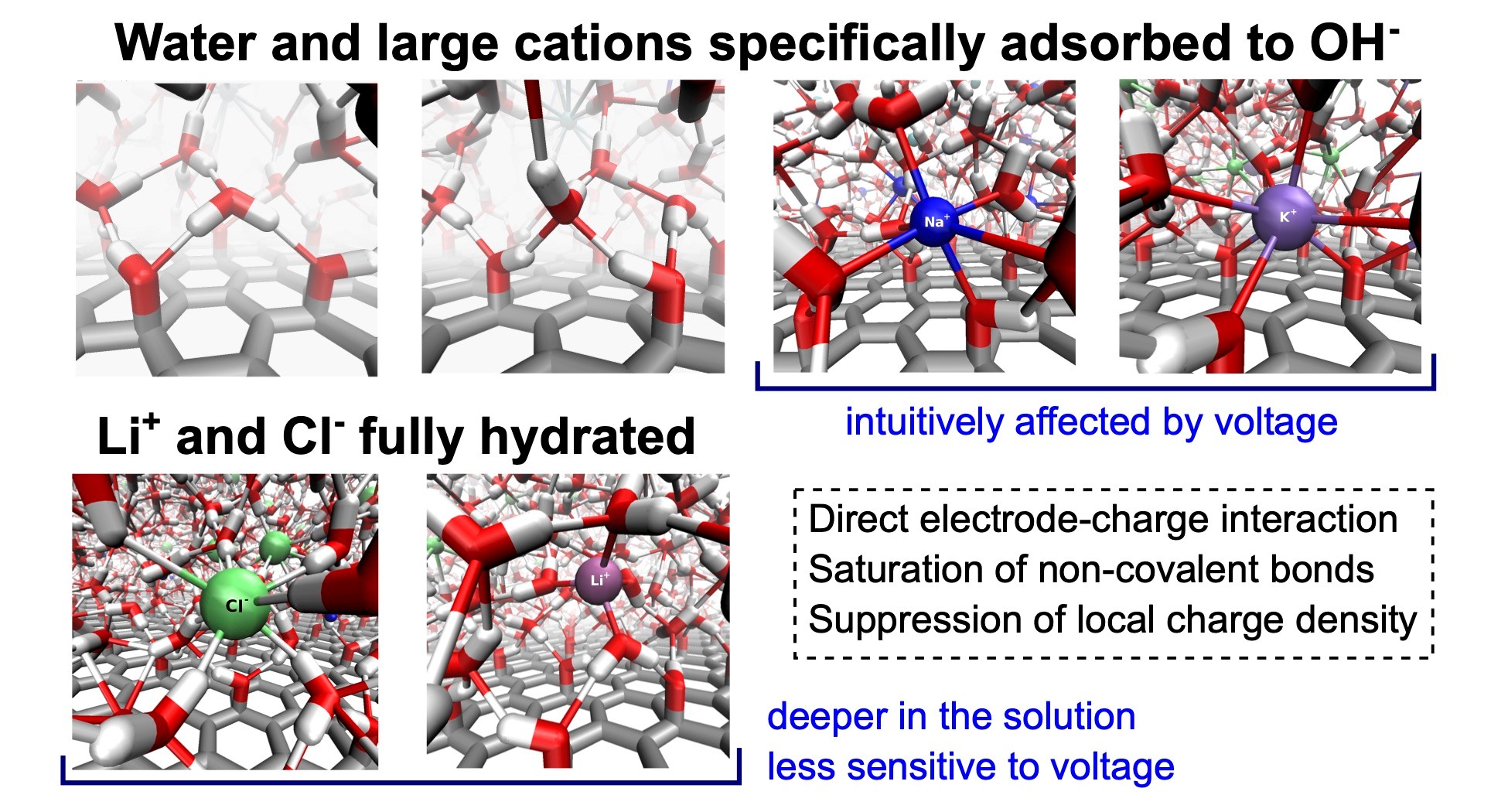
Graphene oxide (GO) is a promising material that finds use in electrochemical applications. Therefore, understanding the microscopic behavior of electrolytes in contact with GO electrodes is important. In this work, we focus on a detailed description and explanation of the structure, adsorption behavior, and self-diffusion of aqueous solutions of single-salt and mixed-cation alkali metal chlorides in the vicinity of hydroxylated GO electrodes under normal thermodynamic conditions and varying interelectrode voltages. We performed molecular dynamics simulations of the solutions constrained between planar GO electrodes using the constant potential method. We analyzed several structural properties, including profiles of atomic density, charge density, characteristics of the network of non-covalent bonds, and in-plane and transverse self-diffusion, all as functions of the distance from the GO surface. We discuss and explain the behavior of all these properties in detail as a result of three driving forces: (i) the direct electrode-solution interactions, (ii) the tendency of the solutions to saturate the network of non-covalent bonds, and (iii) the tendency of the system to suppress local charge accumulation in any region larger than typical interparticle distances. The existence of hydroxyl groups here greatly enhances the direct electrode-solution interactions, causing qualitative differences in the structure, including a different arrangement of the adsorption layers of ions in comparison to the solutions at graphene electrodes.
- J. Dočkal, E. Rezlerová, M. Předota, M. Lísal, F. Moučka: Understanding interfacial structure and preferential adsorption in mixed alkali-halide electrolytes at graphene oxide electrodes by constant potential molecular dynamics simulations. J. Mol. Liq. 424, 127078, 2025. DOI